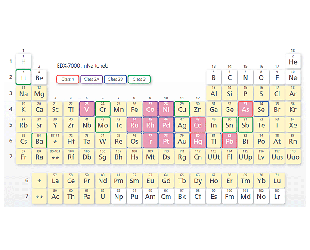
The pink grid squares indicate elements that can be accommodated by the Pharmaceuticals Impurities Analysis Method Package (optional).
Pharmaceutical Elemental Impurities Analysis System
Energy Dispersive X-ray Fluorescence Spectrometer
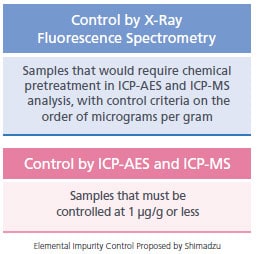
Control of Elemental Impurities in Pharmaceuticals In the pharmaceutical industry, the analysis of elemental impurities is necessary to ensure the safety of pharmaceuticals. In December 2014, the "Guideline for Elemental Impurities" (Q3D) was issued by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), consisting of representatives from Europe, the U.S. and Japan. In Japan, the "Guideline for Elemental Impurities in Drug Products" (PFSB/ELD Notification 0930 #4 from the Ministry of Health, Labour and Welfare) was issued, and will be applied to new drug products submitted for approval after April 1 2017. For 24 elements categorized in Class 1 to Class 3, residual quantities in pharmaceutical drug products must be controlled within permissible limits. Although ICP-AES and ICP-MS are used for precise analysis of elemental impurities, X-ray fluorescence spectrometers can be used as an alternative analysis method. This is because they can quantitatively and qualitatively analyze a variety of elements nondestructively, and without chemical pretreatment, unlike ICP-AES and ICP-MS systems. The X-ray fluorescence spectrometry has been adopted as a general method of analysis in the U.S Pharmacopeia and the European Pharmacopoeia. (USP<735>, Ph.Eur.2.2.37)
Element Classification (24 Elements)
- Class 1:Very toxic. Highly toxic in all administration routes.
- Class 2:Toxic, although it depends somewhat on the administration route.
- Class 2A:Assessment is required in all cases.
- Class 2AB:Assessment is required only when it is intentionally added in a process.
- Class 3:Toxicity is low in oral administration. Assessment is required for other administration routes.
PCEDX Pro Software
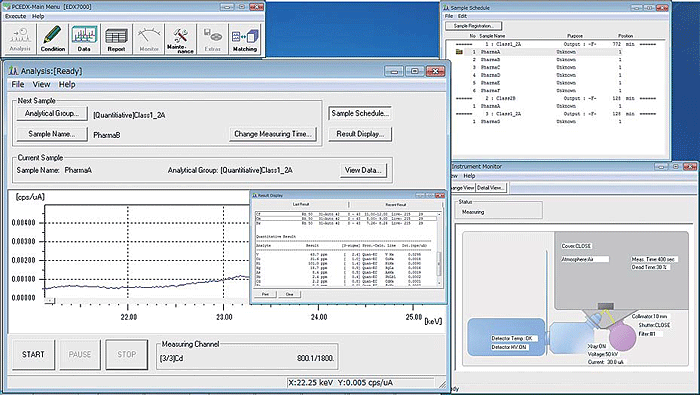
PCEDX Pro Software
Analysis is performed using PCEDX Pro software. Easy-to-use operations enable fully automatic measurements, so even novices can feel confident. This highly functional software supports many quantitative calculations, including the calibration curve method, the fundamental parameters (FP) method, the thin film FP method, and the background FP method (patented by Shimadzu).
Features
-
An optional vacuum measurement unit or helium purge unit is required to measure light elements (15P and below) with the EDX-7000.
The pink grid squares indicate elements that can be accommodated by the Pharmaceuticals Impurities Analysis Method Package (optional). -
The optical system is a bottom exposure type in which both the X-ray tube and the detector are built into the bottom of the instrument. Samples simply need to be placed in the measurement area (the part with the hole) within the sample chamber. The instrument is so designed that the shutter on the X-ray tube will not open...
-
When analyzing powder and liquid samples that cannot be positioned as is in the measurement area, place the sample in a sample cell covered with a special film for X-ray fluorescence. Then start analysis by irradiating the sample with X-rays through the film.
-
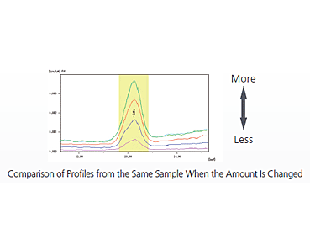
With the heavy elements included in organic substances, even fluorescent X-rays generated from deep in the sample penetrate the sample and reach the detector. As a result, the intensity of the fluorescent X-ray changes depending on differences in the sample amount (sample depth).
News / Events
-
Three Analytical and Measuring Instruments Win the Internationally Recognized “iF Design Award 2024”
The Shimadzu LCMS-2050 high-performance liquid chromatograph mass spectrometer, the Brevis GC-2050 gas chromatograph, and the ICPMS-2040/2050 ICP mass spectrometer won the internationally recognized iF Design Award 2024 in the product design category.
-
New Video: The Cabbage Core, Trash or Treasure?
Most people throw out the cabbage core when cooking, but is the core really just trash or actually a hidden treasure of nutrients?
-
New Video: ICPMS-2040/2050 ProActive Rinsing
ProActive Rinsing can shorten the analysis time by sharting the rinsing sequence in advance. While measuring multiple samples, the rinsing sequence can be started early by sending the autosampler probe to rinse while collecting data using sample already in the suction line. This greatly reduces measurement time and conserves sample.
-
New Video: ICPMS-2040/2050 Extended Rinsing
The extended rinsing function automatically performs an additional rinse sequence when a target element exceeds a predetermined upper limit. A second rinse solution can be used in the additional rinsing sequence to improve rinsing effectiveness. Consequently, carryover is eliminated to ensure high-quality data.
-
Shimadzu has released the ICPMS-2040 Series / ICPMS-2050 Series, Inductively Coupled Plasma Mass Spectrometry
ICPMS-2040/ 2050 Series has achieved a harmonious blend of environmental-friendliness and analytical performance.
-
The Shimadzu LCMS-2050 High-Performance Liquid Chromatograph Mass Spectrometer and the AA-7800 series of atomic absorption spectrophotometers have received the Red Dot Design Award 2023.
The Shimadzu LCMS-2050 High-Performance Liquid Chromatograph Mass Spectrometer and the AA-7800 series of atomic absorption spectrophotometers have received the Red Dot Design Award 2023.