Mass of Materials
Materials are composed of many molecules, which are the smallest substances known for determining the chemical features of the materials. Molecules are further decomposed into atoms or elements. At present, about 110 kinds of elements are known to exist in nature. For example, water molecule H2O is composed of two hydrogen (H) atoms and one oxygen (O) atom.
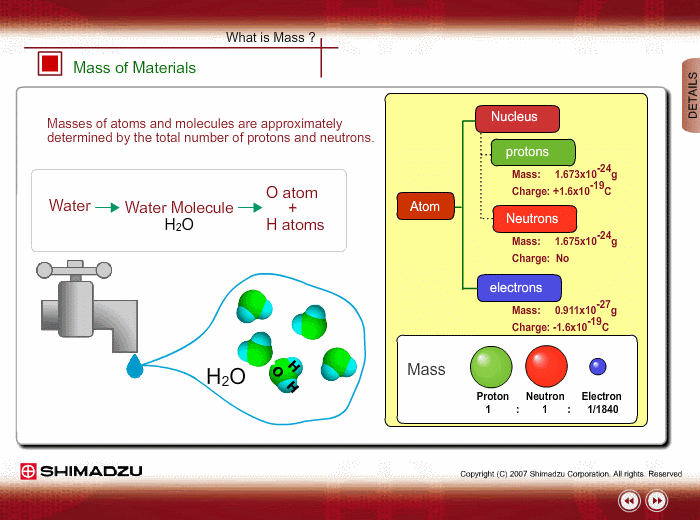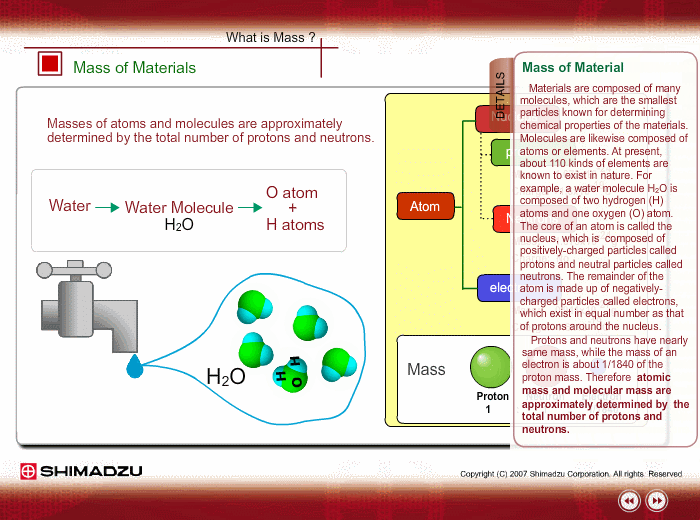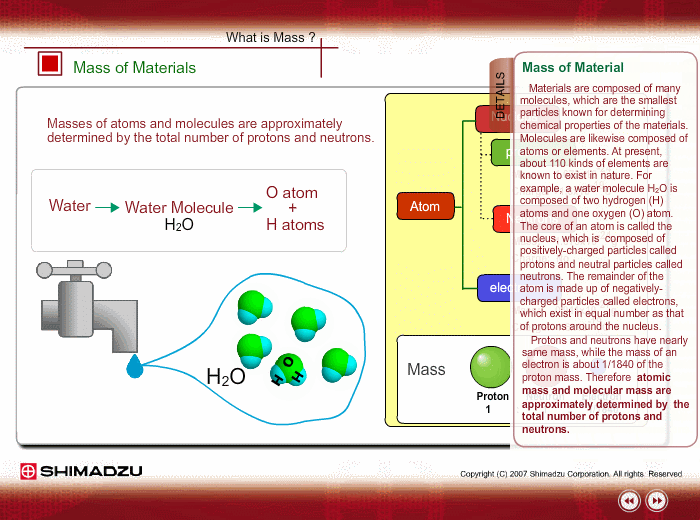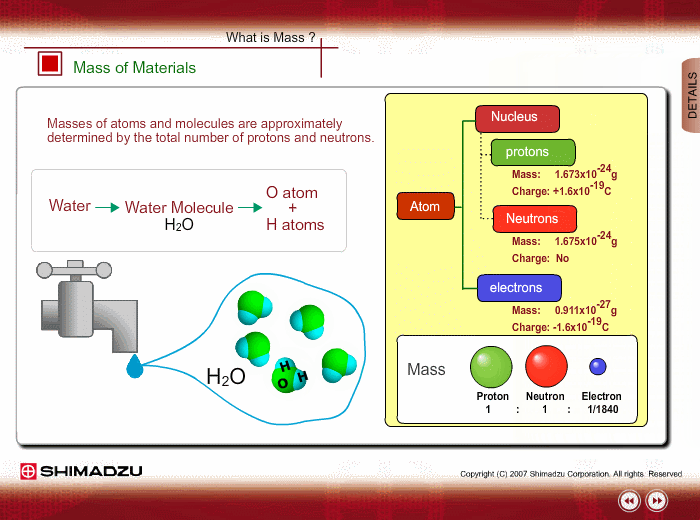
The core of an atom is called the nucleus, composed of protons and neutrons, which exist in equal number around the nucleus
Protons and neutrons have nearly same mass; meanwhile, the electron mass is about 1/1840 of the proton mass. Therefore, atomic mass and molecular mass are approximately determined by the total number of protons and neutrons.
Next >> Mass Number and Isotope


