Cleaning Validation
What is cleaning validation?
Cleaning validation, which is essential for quality assurance in pharmaceutical manufacturing, refers to the quantification of residues after cleaning of manufacturing facilities and equipment to verify that the amount is below a predetermined acceptable limit.
- Regular equipment cleaning and post-cleaning validation are required to prevent cross-contamination and contamination of products.
- Even in the case of exclusive facilities for the same product on the same line, regular cleaning is necessary due to risks of contamination.
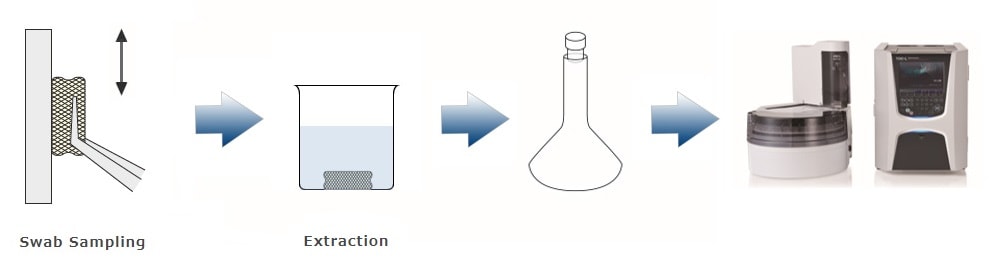
Generally, total organic carbon (TOC) analysis and high performance liquid chromatography (HPLC) are the verification methods. As shown in the table below, each technique has its own characteristics, and it is necessary to select the one suitable for the circumstances and the target for verification. This page introduces the application of TOC analysis, which enables simple, rapid measurements and can detect all organic substances.
| Technique | Features |
|---|---|
| Total Organic Carbon (TOC) Analysis |
|
| High Performance Liquid Chromatography (HPLC) |
|
Evaluation method using a TOC analyzer
TOC analyzers are mainly used for water quality analysis of liquid samples such as tap water, environmental water, and waste water. In addition, installing special accessories makes it possible to measure nitrogen components in liquid samples and carbon components in solid samples. Because of this expandability, TOC analyzers have recently been introduced not only for pharmaceutical products but also for cleaning validation at cosmetics and food manufacturing sites. They are also used in the manufacturing process and quality evaluation of various medical devices to evaluate the cleanliness of products after cleaning.
The following three methods can be proposed for cleaning validation using a TOC analyzer. The method used depends on the material, size, and shape of the equipment or products subject to cleaning validation. Our TOC analyzer, configured with the appropriate accessories, supports each method, making it applicable to a wide range of environments.
3. Swab Sampling - Direct Combustion Carbon Measuring Method
The residue is wiped and the swab material is inserted directly into the heating section. There is no need for solvent extraction, so the water-insoluble residue can be measured reliably. Although total carbon (TC) is measured by this method, it can be considered equivalent to TOC measurement in the sense that it is judged to be below the acceptable limit.
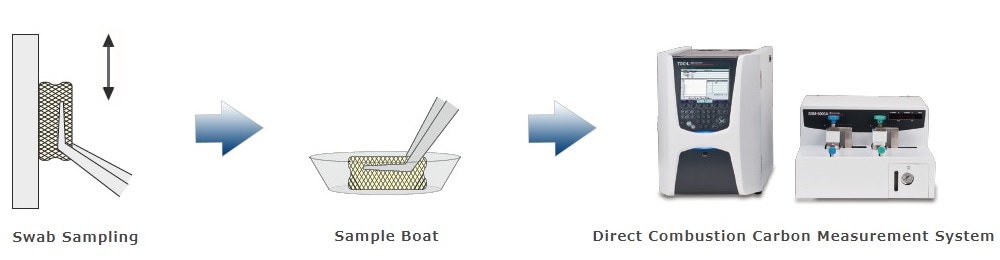
The swab sampling method is recommended by the FDA. Our proposed swab sampling-direct combustion carbon measuring method does not require extraction, so it can take advantage of the benefits of swab sampling. The following is a detailed procedure.
Procedure for Swab Sampling - Direct Combustion Carbon Measuring Method

Swab Material: "Easy wiper-S"

The "Easy wiper-S" is available for swab sampling. It has the following features.
- Fibrous, soft and easy to wipe
- Efficiently samples hard-to-get swab debris from the wiping surface
- Easy to hold and put in the sample boat
Setting Residual Acceptable Standards
There are several approaches to the residual acceptable standards, such as NOEL (no-effect level), 0.1% standard, and 10ppm standard. Using these as a reference, it is necessary to set appropriate standards in each company.
News / Events
-
The TOC 50th anniversary special website has been opened.
The website has been established to explain the basic knowledge about TOC and describe the history of Shimadzu TOC analyzers.
-
Science and Technology for Soil Preratation
We interviewed Prof. Kubo, who has been developing and disseminating the ”Soil fertility diagnosis: SOFIX [Soil Fertile Index]”, a method for visualizing soil preparation by analyzing biological properties, a goal which has been difficult to achieve with conventional technologies.
-
Shimadzu has released the Certified Pre-Cleaned Vials for Total Organic Carbon Analyzers.
In contrast to conventional vials, Certified Quality (CQ) vial maximize reliability and enable higher workflow efficiency.
-
Shimadzu Enters the Field of On-Line TOC Analyzers for Pure Water with the Mercury-Free TOC-1000e, the First Analyzer in the New eTOC Series