Total Solution for PIC/S and FDA Compliance
Shimadzu Presents PIC/S GMP/FDA 21 CFR Part 11/Computerized System Validation Total Solution
All Shimadzu network system products incorporate functions for the PIC/S GMP and the Part 11 compliance regulation, and computerized system validation functions required by GxP. Shimadzu provides documentation including IQ/OQ, Certificates of Compliance, and Inspection Test Result Reports based on Shimadzu IS09001 certified system. Shimadzu's accredited service personnel offer full support for validation of customer's Shimadzu products. In the latest information on PIC/S and FDA regulations through seminars and workshops, participating in vendor audits demanded by agencies, and actively assisting customers to comply with new regulations.
Shimadzu Global Resources Provide Local Support for PIC/S and FDA Compliance
Shimadzu Total Support for PIC/S GMP and Part 11 Compliance
Shimadzu HPLC, GC, Mass Spectrometers, UV-VIS spectrophotometers and other spectroscopy products and their associated data processing systems all incorporate sophisticated, leading-edge technology for Access control, Audit trail, and Protection and Security of data functions to comply with regulatory demands. In addition to offering instrumentation and network-compatible software, Shimadzu offers total support for creating system control and management procedures, provides information, organizes seminars, and offers post-installation training on PIC/S GMP and Part 11.
| PIC/S refers to the Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme.PIC/S devises and promotes harmonious GMP standards and quality systems for the inspection of medical supplies. There are currently over 50 members of PIC/S, including agencies from theUnited States and EU countries. Japan, South Korea and other countries have applied for affiliation, while China and Russia show interest in being affiliated. PIC/S PIC/S is a private agreement between the regulatory agencies of each member nation. The affiliated member is the agency or authority responsible for inspecting pharmaceutical companies. Activities include the training of inspectors, the networking of the affiliation inspection authorities, and the evaluation of GMP inspection. PIC/S does not inspect a pharmaceutical company directly. It is thought that the GMP standards published by PIC/S will become internationally accepted. |
Shimadzu's Response for Regulatory Compliance
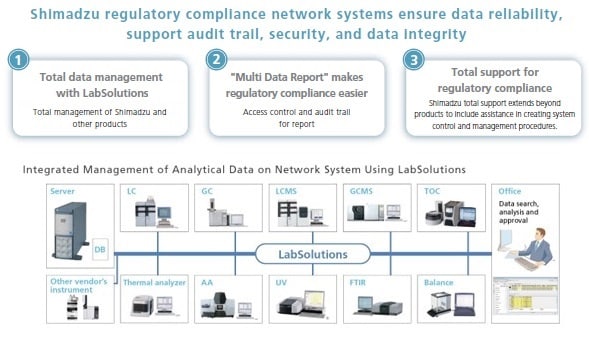
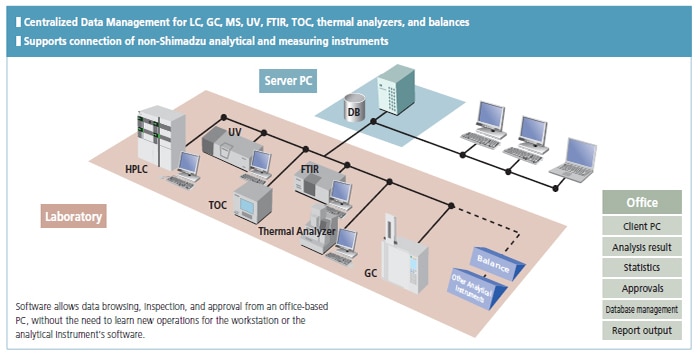
Shimadzu's basic policy is to comply with regulations by integrating data management for all instruments used in the laboratory, including chromatographs and mass spectrometers (HPLC, GC, LC-MS, GC-MS), spectrophotometers (UV, FTIR etc.), total organic carbon analyzers (TOC), thermal analyzers, and balances.
Shimadzu's LabSolutions products provide solutions for the regulatory compliance of all essential laboratory analysis data from chromatographs and spectrophotometers to balances. Shimadzu supports networking for all analytical instruments to enhance workflow efficiency and data reliability.
Computerized System Validation
Computer systems must be validated according to an appropriate procedure to ensure the reliability of electronic records and electronic signatures.
In addition to the functions required for compliance with all regulations including Part 11, the FDA has added basic requirements for the computer system itself such as the number of clients connected to the network, disk capacity, network expandability, etc. When creating specification requirements, it is important to confirm such basic information in addition to standard regulatory items.
Key Points
- Confirm the specification requirements.
- It is important that the specification requirements meet the demands on the system and operating environment, and also incorporate the technical elements to satisfy Part 11.
- The documentation of plans, procedures, and reports and appropriate review, approval, and management.
Shimadzu Regulatory Compliance Network Supporting
Shimadzu Regulatory Compliance Network Supporting Database Management Software LabSolutions Features
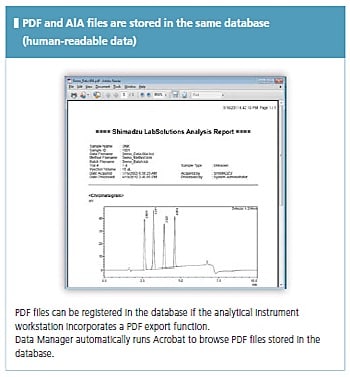
LabSolutions complies with the requirements of the FDA 21 CFR Part 11 (Electronic Records, Electronic Signatures). LabSolutions provides secure data management and electronic signature operations for measurement results registered in a database acquired from a range of instruments, including HPLC, GC, GC-MS, LC-MS, UV, FTIR, and balances, as well as other manufacturers' products. The data is automatically saved in the database for subsequent easy searching. Additionally, the associated method and schedule information, date of measurement, operator's name, and analytical report image files (in PDF format) are stored together. Client/server capability allows centralized management of data from all instruments and simple data referencing from a client PC.
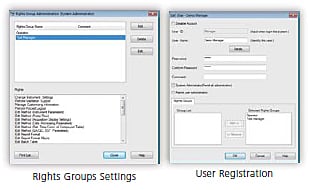
Effective User Management
LabSolutions’ user administration comprises the setting of rights groups and assignment of rights to users just as in Windows. It enables the easy setting of user access rights as well as rights groups matched to each user's required tasks. Functions such as these achieve effective user administration matched to laboratory operations from managerial tasks to data acquisition operations.
Firm Security
Features functions for setting an audit trail to ensure data reliability and formailing events occurring on the system. It includes various settings, such as setting the length, expiration date and complexity of passwords for user accounts, setting the lockout function to prevent illegal access, and registering settings for the deletion and alteration of registered users, to enable highly secure system operation. Settings for overwriting data files and other information and relating to items to output in reports also are supported.
Log Browser for Ascertaining System Operation Status
The Log Browser allows the user to easily ascertain system status from operation and administration to usage status and error status. Functions to search for log details, user names, instrument names, etc. also are provided in order to verify the necessary information.
Audit Trail for Achieving Change History Management
The change history of method files is managed by the audit trail. Application of the audit trail to all methods can also be set in the security policy settings. This prevents inconsistencies in compliance with regulations. A history of postrun analysis after data acquisition can also be managed by applying the audit trail to data files.
Shimadzu Multi Data Report Achieves Regulatory Compliance
Multi Data Report solves the Excel problems

1. Audit trail problems
・Difficult to identify the creator, date and time of registered data
・Impossible to know who changed values or formula and when
2. Data security and integrity problems
・No means to prevent overwriting or deleting files
3. Electronic signature problems
・No electronic signature functions available
Features of Multi Data Reports
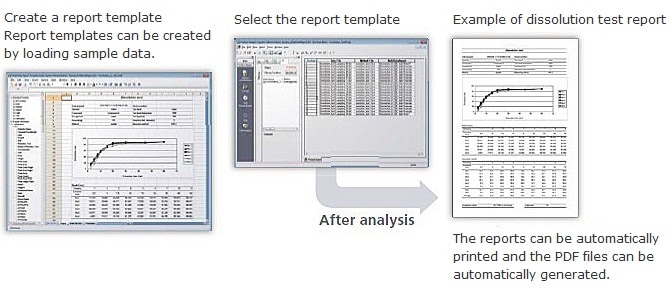
Simple system creation
Easily create report templates as flexible as Excel sheets by visual operation. Created templates are easily registered to the database for secure management using the registration tool. This reduces the workload before operation of the system.
Easier report generation
Generate reports automatically by simply selecting analysis data and report templates registered in the database.
In addition, reports are created simultaneously when an analysis ends using the analysis schedule. No manual input is required, saving effort for report generation and checking. Verified templates for system suitability tests, content uniformity tests, and other standard tests are provided as standard.
Automatic generation of PDF files
A PDF file is generated automatically at the same time a report file is created. The excellent portability of PDF files is effective for electronic report transfer.
Automatic database registration
Created reports are automatically saved in the database for secure storage. Required reports and analysis data can be easily found and recalled using the associated information that is automatically registered simultaneously.
Electronic signature-compatible
Electronic signatures can be applied to reports using standard functions of LabSolutions Data Manager.
Automatic Report Generation by Batch Analysis
Multi Data Report Meets All Regulatory Requirements
"Multi Data Report" registers pre-created templates to a database. When a report is generated, it automatically selects the template, pulls required data from the database and conducts the calculations. Through this procedure, only templates and data already in the database are used for calculations.
The report that calculated the results is also saved and managed in the database. Consequently, the user does not directly touch the data, thereby maintaining the access control, audit trail, and protection and security of data.
| Access control | Templates are saved in a secure database. |
| Audit trail | Database records secure audit trail. Possible to apply electronic signatures on reports. |
| Protection and security of data | Data in database cannot be changed. |
| Validation | No macros are required, only sheet functions are used, making validation simple. Validated templates are controlled in a database. |
| PDF report | Reports are automatically created as PDF files. |
| Easy to use | Transcription of data is not required. This eliminates the time needed to re-check data to prevent errors in transcription. |
Enabling Report Creation for Various Instruments
LabSolutions' Data Manager can prepare integrated reports using analysis data and results obtained from various instruments. Not only can reports be created for HPLC, GC, MS, FTIR, UV, balances, and other instruments individually, but they can include combined data from several instruments.
Enables automatic printing of prepared reports and automatic generation of PDF files.
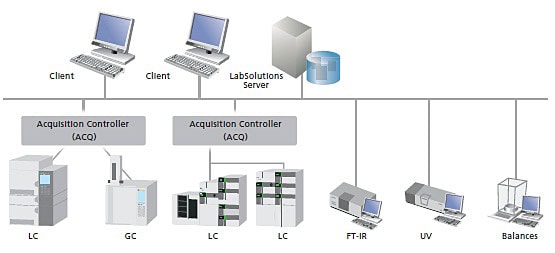
Shimadzu Chromatography Data System - Offering customers optimal solutions
Shimadzu's LabSolutions Chromatography Data System is the unified operation software. From testing analytical conditions to analyzing data and creating reports, it is designed so a wide variety of functions can be accomplished using simple operations. For validation, it is even flexible enough to comply with the increasingly important database management and networking requirements.
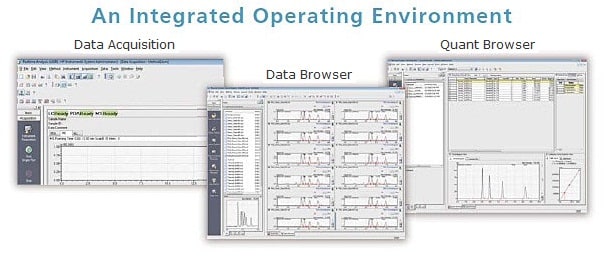

Features of LabSolutions
- Simultaneous control of HPLC and GC Systems
- Easy operation and more efficient analysis work using the quant browser and data browser
- Report with a high level of freedom from various analyses to summary reports
- Flexibility to expand from a standalone to a network system
- GxP compliant validation support functions
- GxP and Part 11 compliant user and data management features
LabSolutions CS
Freely Accessible to the Analysis Network
Since all analytical data are managed in the database of a server computer, LabSolutions CS can read data from any personal computer on a network. In addition, analysis directions and instrument monitoring and control can be performed from a personal computer (client PC) not connected to the instruments.
Moreover, client PC functions are performed on a server and client PCs corresponding to a Windows terminal service do not need to install LabSolutions software. Furthermore, LabSolutions CS corresponds to Citrix XenApp and can perform more advanced server management.
Recommended for the following customers
- Facilities with a large number of instruments and users
- Facilities interested in enhancing procedural efficiency
- Facilities interested in enhancing managerial efficiency
- Facilities where existing PCs can be used as client PCs
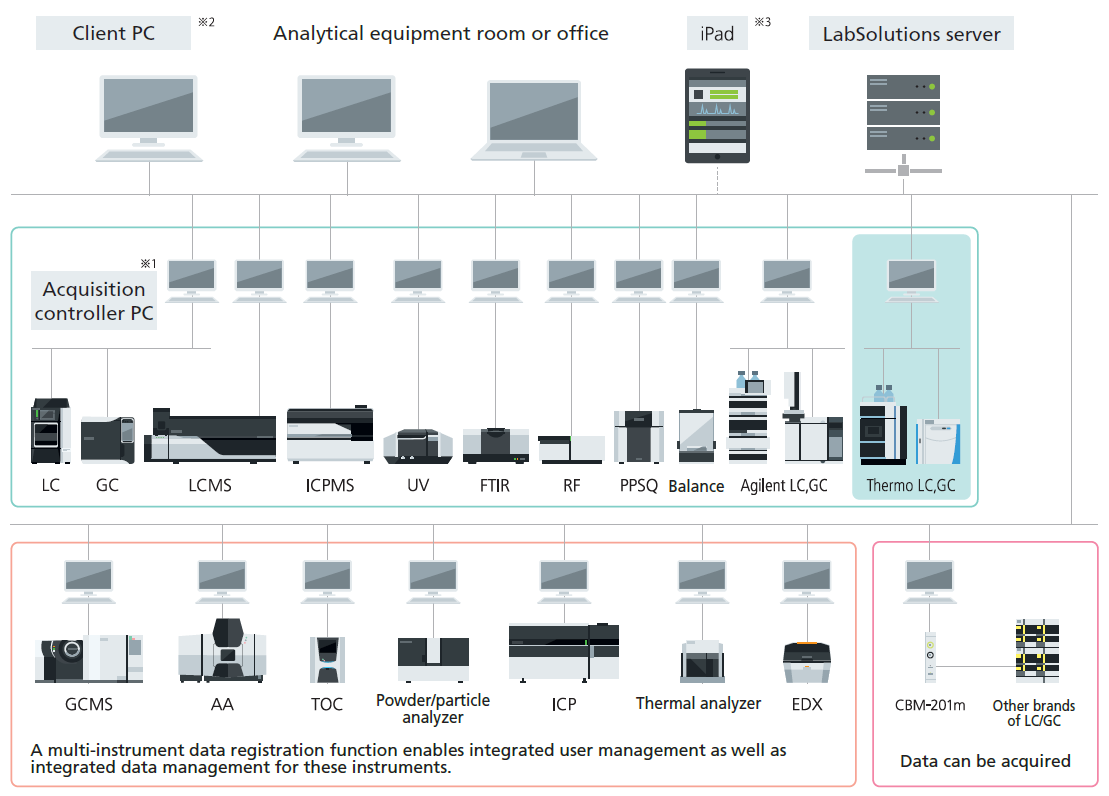
Note 1: The acquisition controller PC is used to control the analytical instruments.
Note 2: If a terminal service is used, the LabSolutions software does not need to be installed on the client PC.
Note 3: If an iPad is used, the Citrix XenApp must be installed.
Full Support for Regulatory Compliance
Installation and IQOQ Inspection
- IQOQ is performed by field engineers certified by Shimadzu Corporation to ensure secure inspection.
- A standardized inspection protocol allows engineers to effectively perform an IQOQ in a short time.
- This service reduces space for storage of documents and improves visibility of inspection results.
- Currently this service supports HPLC, UV and TOC. Other instruments will be supported sequentially.

Provides Summary Report of IQ/OQ Inspection Results
Customers are provided with a summary report of installation information recorded for IQ, such as the serial and model numbers of instruments included in the IQ process, as well as setting values, control criteria, and pass/fail results for each OQ inspection parameter. This summary report helps ensure that customers can confirm inspection results and the regulatory authority can audit efficiently.

Example of IQ/OQ Inspection Results
Summary Report Output
Total support as described is available in certain countries. Please contact your local Shimadzu representative for clarification and details.
News / Events
-
Shimadzu has released the LabSolutions i-QLinks.
LabSolutions™ i-QLinks provides integrated control over the quality testing operations of an analytical laboratory, including the preparation of test plans and instructions, the incorporation of test results from analytical instruments such as HPLC, the automatic preparation of test reports from the incorporated test results, and the management of the quality test progress.
-
Shimadzu has released the LabSolutions Sync
Synchronizes Third-Party Software for Pretreatment Units with Shimadzu LC and LC-MS
-
Efficient Method Development by automated pH Screening with LabSolutions MD
This article describes an example of using LabSolutions MD, a dedicated software for supporting method development, to automate pH screening by varying the mobile phase pH from 2.5 to 8.5 to evaluate the optimal pH level for separating 12 small-molecule drugs.
-
Release of Six New AP-AD Series Analytical Balances
The new AP-AD series models not only offer excellent basic performance, such as fast weighing times of about two seconds and high measurement stability that minimizes errors, but also include new functionality that improves convenience, such as automatic doors and touchless sensors.
-
Shimadzu has released the LabSolutions DB GCMS for GCMS-QP series
GC/MS software for secure database management in compliance with regulations.
-
What you should know to assure laboratory data integrity with LabSolutions


