Setting the Fraction Volume Target
— To Select the Appropriate Instrument —
When fractioning target components by HPLC, for the sake of subsequent processing, it is important to be able to specify the fraction volume or at least the minimum meaningful fraction volume. For example, a specialized fraction collector is not required if a fraction volume in the order of micrograms is adequate. This fractioning may be possible using a conventional size of instrument and column. Conversely, if a fraction volume in the order of grams is required, a preparative scale of instrument and column is probably needed. Therefore, the instrument and column selection is significantly affected by the fraction volume required.
(1) Understanding the Current Status at Conventional Scale
First, we calculate the volume that can be fractioned on a conventional scale (using a normal HPLC instrument and column). An understanding of the fraction volume at a conventional scale permits selection of the appropriate preparative HPLC to obtain the target fraction volume. (This assumption is based on HPLC analysis conditions that have been established to measure the concentration of the analysis target components in the actual sample, and that the eluent used does not cause any problems during post-fractioning processing.)
■ Calculating the absolute injection volume
If the concentration of the target component in the sample solution and the volume injected into the HPLC are both known, they can be multiplied together to determine the absolute injection volume. (For example, if the concentration of the target component in the sample solution is 1 mg/L and the injected volume is 10 µL, the absolute injection volume is 0.01 µg.) The most commonly measured concentration range by HPLC with a UV detector extends from sub-mg/L to several hundred mg/L. It is easy to see that fraction volumes of just a few micrograms can be obtained by fractioning with such an instrument. Obtaining a 1 mg (1000 µg) fraction requires the injection of 100 µL of a 1 % (10,000 mg/L) sample solution.
■ Determining the load limit
Next, it is recommended to determine how high the analysis target concentration can be increased in the sample solution and how much the injection volume can be increased using a conventional-scale instrument. The diagrams below show examples of test results.
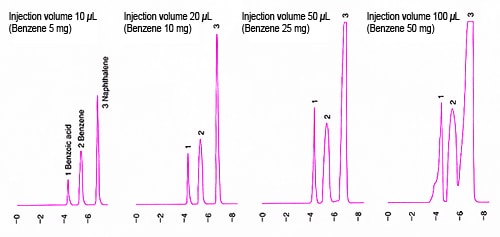
* To prevent the peaks for high-concentration benzene going off-scale, detection was performed at 270 nm to avoid the maximum absorbance.
This example shows chromatograms of a mixed solution of 500 mg/mL benzene with trace levels of benzoic acid and naphthalene analyzed using a conventional-sized ODS column (250 mm × 4.6 mm, 5 µm). The results of these tests show that the peak shape can be maintained up to 50 µL injection volume, which corresponds to 25 mg when converted to a benzene load.
The tests above were performed while increasing the injected volume. However, it is also possible to gradually increase the sample concentration. As a detailed investigation is not required, the injection volume or sample concentration can be increased in steps of 5 or 10 times to determine the limit to which the target component peak can be separated from the adjacent impurity peaks. It is important to determine only whether preparative LC can separate the target peak from coexisting peaks. It does not matter if the peak shape is somewhat deformed or the detector goes off-scale.
If the load limit is known at the conventional scale, it is possible to calculate how much the fraction volume can be increased by scaling up the column size.
(2) Predicting Scaling Up
Once the fraction volume has been established at the conventional scale, it can be compared with the fraction volume target value.
1) Target approximately equals the fraction volume at the conventional scale.
| → | Specialized fraction collector or larger column is not required. If the fraction volume is slightly below the target value, repeat fractioning several times. |
2) Target is 10 to 100 times the fraction volume that can be obtained at the conventional scale.
| → | Introduce laboratory preparative HPLC to achieve the target. Select the appropriate instrument and column to introduce laboratory preparative HPLC. |
3) Target is more than several hundred times the fraction volume that can be obtained at the conventional scale.
| → | Target may be difficult to achieve with a laboratory HPLC instrument. Consider an industrial preparative LC or use a separation method using a different principle from HPLC. |


