Open Solution
Open Access Software for LC and LCMS
Optional Open Access Software for Analytical and Purification
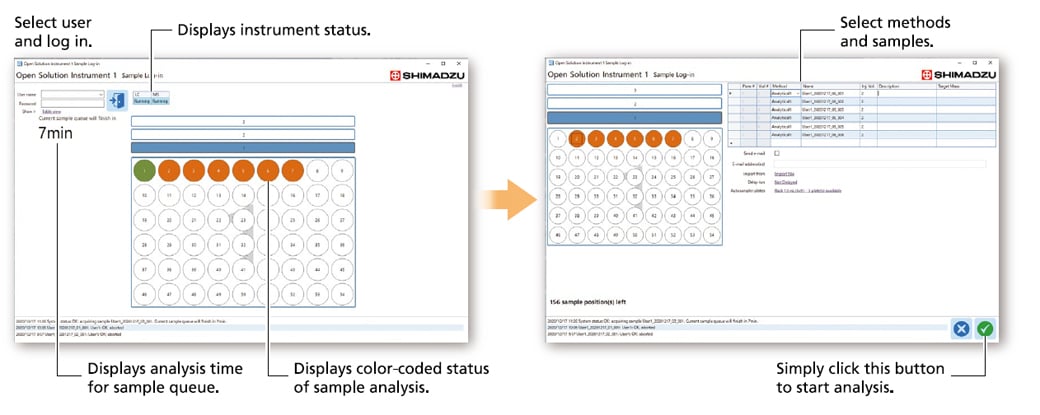
Simple and Intuitive Sample Logging and Data Review
- After logging in to the [Sample Log-in] window, simply select methods and samples to start analysis.
- Open-access functionality for sharing instruments among multiple users.
- Performs automated method switching for multiple users, including column and mobile phase switching, and automatic rinsing of flow lines.
- LC, PDA and MS instrument status.
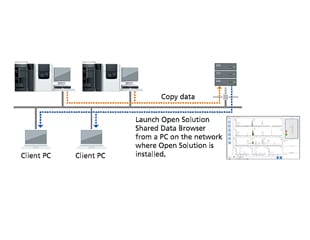
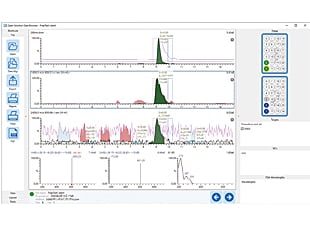
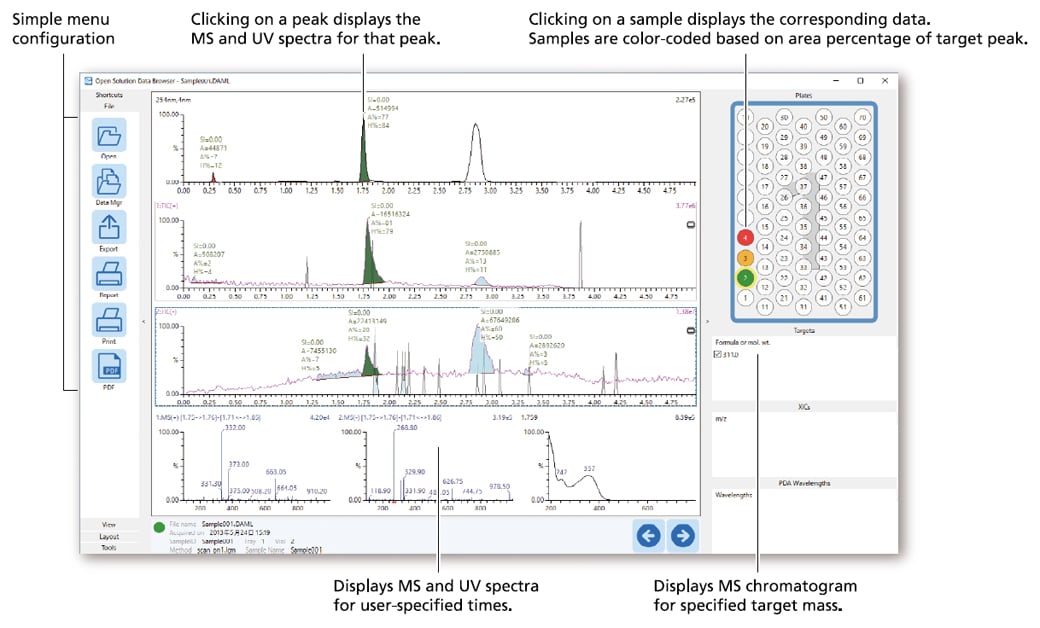
Data Browser—Display and Analyze Data
- Quickly display data by simply clicking on samples in the rack diagram.
- The data browser can be launched on any networked computer by simply installing software on that computer.
- Peak integration (adding or deleting peaks) for LC chromatograms can be performed easily.
- Displays MS and UV spectra for user-specified times.
- Calculates and displays peak purity based on similarity score of MS spectrum.
Features
-
-
Supports Reverse Phase Preparative Scale-Up
News / Events
-
Three Analytical and Measuring Instruments Win the Internationally Recognized “iF Design Award 2024”
The Shimadzu LCMS-2050 high-performance liquid chromatograph mass spectrometer, the Brevis GC-2050 gas chromatograph, and the ICPMS-2040/2050 ICP mass spectrometer won the internationally recognized iF Design Award 2024 in the product design category.
-
GCMS-QP2050 Gas Chromatograph Mass Spectrometer Video has released
The business environments and needs involved in analysis work change on a continual basis. The next-generation GCMS-QP2050 gas chromatograph mass spectrometer, with its accumulation of impressive Shimadzu technology, will lead the way forward. New value is provided by hardware boasting astounding reliability and stability, and easy-to-operate software equipped with superior automated technology.
-
OAD-TOF system Interview with Dr.Hiroshi Tsugawa
The OAD-TOF system is a Q-TOF LCMS that realizes OAD (Oxygen Attachment Dissociation), Shimadzu's proprietary fragmentation technology. It allows the analysis of the position of carbon-carbon double bonds in lipids and other organic compounds. Combined with the LCMS -9050, which boasts world-class high mass accuracy and fast polarity switching, it enables the creation of innovative applications.
-
Metabolic Pathway Analysis Solutions
Metabolic pathways are the chain of chemical and enzymatic reactions that occur within a cell in living organisms to support their life.
-
AOAC INTERNATIONAL Posters are now available.
-
Shimadzu has released the OAD-TOF system
The OAD-TOF system is a Q-TOF LCMS that realizes OAD (Oxygen Attachment Dissociation), Shimadzu's proprietary fragmentation technology. It allows the analysis of the position of carbon-carbon double bonds in lipids and other organic compounds. Combined with the LCMS-9050, which boasts world-class high mass accuracy and fast polarity switching, it enables the creation of innovative applications.