Baseline Stabilization When Using Refractive Index Detectors
Refractive index detectors (RID) are widely used for many substances, such as to analyze components with low UV absorption, but it requires time for the baseline to stabilize, which has no doubt been a source of frustration for many users. This page discusses know-how regarding baseline stabilization by focusing on non-aqueous size exclusion chromatography (SEC), using mainly tetrahydrofuran (THF) as an eluent.
Adjusting the Detector Temperature
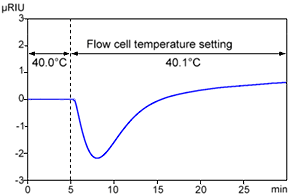
Change in Refractive Index in Response to Change in Cell Temperature
The refractive index is affected significantly by the solution temperature. Therefore, many commercially available RIDs are designed to control the temperature around the flow cell to ensure its temperature remains as constant as possible. So, how much do tiny variations in detector temperature affect the baseline?
Figure 1 shows an example of data obtained when the flow cell temperature was changed from 40.0 °C to 40.1 °C. Though only a temperature difference of 0.1 °C, it clearly causes the baseline to fluctuate. Presumably this occurred due to a difference in heating rate between the sample flow cell, with eluent flowing through it and the reference cell, with eluent trapped inside.
In actuality, the flow cell is temperature-controlled, so the baseline would not fluctuate this much if the laboratory temperature changed by only 0.1 °C. However, if the room temperature suddenly changes or the HVAC system blows air on the detector, the temperature control system in the detector sometimes cannot keep up with such changes. Therefore, measures should be taken to keep the room temperature as constant as possible, or to wrap the flow lines entering the detector with insulation.
Adjusting Dissolved Air Content in Eluents
One of the characteristics of RIDs is that they also detect dissolved air in eluents. Therefore, to stabilize the baseline, it is necessary to implement measures to ensure the degassing state of the eluent remains constant. Figure 2 compares the size of baseline fluctuations, in results obtained using water and THF as the eluent, when the degassing unit was switched ON after the baseline had stabilized with the degassing unit OFF.
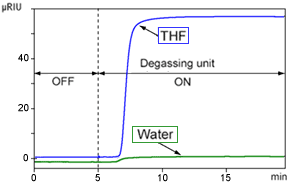
Change in Refractive Index When degassing unit Is Switched from OFF to ON With no column, 1.0 mL/min flowrate, and DGU- 20A3 degassing unit
This graph shows two things.
1) Decreasing the dissolved air in the eluent increases the refractive index
2) The change in refractive index is greater for THF than for water.
Point 1) indicates the opposite trend as the UV detection near 200 nm. For UV detection, a higher level of dissolved air normally increases the absorption level.
Point 2) indicates that, in general, the lower the polarity of a solvent, the greater the effect a change in dissolved air has on the refractive index. This is because non-polar solvents dissolve air more readily than polar solvents (see: Mechanism of How Air Bubbles Are Generated), which results in a larger change in dissolved air after the degassing unit is switched ON.
Those who have experience with both aqueous and non-aqueous SEC probably have more difficulty with baseline stabilization in non-aqueous SEC than in aqueous SEC. In non-aqueous SEC, detecting the refractive index (or detecting the UV absorption near 200 nm) requires particular care in controlling the dissolved air level.
The DGU-20A3 degassing unit used in this case uses a gas-liquid separation membrane* with a higher air permeability than previous models and flow lines with about 1/20 the volume of previous models. This significantly reduces the time required to reach equilibrium after degassing starts.
|
* |
Due to flow line materials, some solvents cannot be used in this system, such as hexafluoroisopropanol. Carefully select the solvents used in the eluent. |
Also, be aware that excessive offline degassing, such as with a vacuum degassing unit that uses an aspirator, can result in air gradually redissolving into the eluent after offline degassing, such that the changing dissolved air content prevents the baseline from stabilizing.
Adjusting the Temperature of Eluents
Figure 3 shows the results from immersing the eluent bottle in a water bath and recording the baseline fluctuations as it is heated from 25 °C to 35 °C.
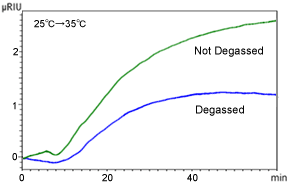
Change in Refractive Index in Response to Change in Eluent Bottle Temperature Without a column, eluent: THF, and flowrate: 1.0 mL/min
Even if the RID and column oven are kept at a constant temperature, simply changing the temperature of the eluent bottle can cause the baseline to fluctuate. One reason is presumably that the amount of air dissolved varies depending on the liquid temperature. The higher the temperature of liquids, the more difficult it is for air to dissolve in them. Therefore, heating the eluent reduces the dissolved air content, which results in increasing the refractive index.
This effect can be inhibited somewhat by using an efficient degassing unit, but if the baseline does not stabilize, implement measures to maintain not only the column and detector temperature, but to keep the eluent bottle temperature constant as well. Also, start analyses after liquids have adjusted to room temperature and wrap the eluent bottle with insulation.
Inhibiting Change of Composition in Eluents
Since the composition of THF and chloroform can change when exposed to air for long periods, due to oxidation or other factors, a stabilizer is added to prevent such changes. In many cases, butylated hydroxytoluene (BHT) is added as the stabilizer in reagent grade THF. In contrast, HPLC-grade THF is sold without a stabilizer and sealed in nitrogen gas, due to the strong UV absorption of BHT and other factors. So, which reagent should you choose?
In general, if using a detector where detecting BHT is likely to interfere with the measurement, then a reagent without the stabilizer should be selected. On the other hand, since the stabilizer inhibits changes in the THF composition, it is probably appropriate to select the reagent with stabilizer whenever the BHT does not interfere directly with measurements.
This is summarized in Table 1.
Table 1 THF With/Without Stabilizer Versus Detectors
Detectors for Which a Reagent Containing a Stabilizer Should Be Used
- Refractive index detectors
Detectors for Which a Reagent Without a Stabilizer Should Be Used
- UV detectors (especially for shorter wavelengths near 200 nm)
- Evaporative light scattering detectors
- LC/MS and LCMS/MS systems
If the reagent without stabilizer is used, be sure to record the date the reagent bottle was opened and be aware of the elapsed time since opening. Once the solvent is opened, ideally it should be stored shielded from light in an airtight container filled with nitrogen. If a series of analyses will extend over a long period, the eluent bottle should probably be kept slightly pressurized with nitrogen gas, even during the analyses. (For more details, contact the reagent manufacturer.) Rather than purchasing more THF than needed, we recommend purchasing smaller quantities more frequently, even if that is slightly more expensive.
Furthermore, during humid weather, baseline fluctuations caused by the absorption of moisture can be inhibited by installing a dehumidifying tube filled with silica gel in the eluent bottle opening. (Gt)


