Q: According to documentation, methanol has a higher elution capacity than acetonitrile. Are there cases where this is not true?
A:
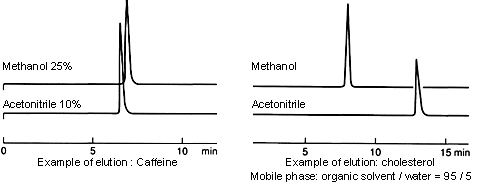
Organic solvents are often used in combination with water for HPLC mobile phases. The elution capacity depends on the proportion of organic solvent in the mobile phase.
In many cases, the elution capacity of methanol/water mobile phases is less than that of acetonitrile/water mobile phases. When switching from an acetonitrile/water mobile phase to a methanol/water mobile phase, use a proportion of methanol that is greater than that of the acetonitrile. (For example, if the proportion of acetonitrile in the mobile phase is 50%, use an 80% proportion of methanol.)
On the other hand, if the proportion of organic solvent is equal or very close to 100%, the intrinsic properties (e.g., the polarity) of the solvent seem to become more prominent, and the elution capacity of methanol is greater.