STM Observation of Ni, Co and Pt Surface Morphology Changes due to CO Blowing (2)
Introduction
Cobalt (Co) is an iron-group element, like nickel (Ni), and its chemical properties are similar to those of nickel. Following on from the nickel specimen covered in the previous example, here we present an example in which the macro-level changes in surface morphology that occur when a cobalt thin-film surface comes in contact with CO gas are observed using an STM in a gas atmosphere (Shimadzu WET-901 Environment-controlled STM).
We also present the results of the same experiment performed on platinum (Pt), which does not react easily with CO gas. This is the first time for the changes in surface morphology that occur in a specimen to be observed in detail using an STM under Environment-controlled conditions where the specimen is heated in a vacuum and gas is blown onto it.
Real Time Observation of Co Surface Morphology Changes due to CO Blowing
Cobalt is oxidized more readily than nickel in air and so, in order to remove the oxygen at the surface, while the cobalt specimen was heated to approx. 300ºC, H2 gas was blown onto the specimen under vacuum conditions. The specimen was returned to room temperature, retaining the metal surface exposed by this reductive reaction, and CO gas was blown onto the specimen’s surface.
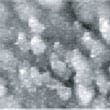
Fig.1 (□185.6nm) Co surface before CO blowing
Fig. 1 shows an image of the cobalt surface obtained with STM observation after reductive processing.
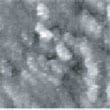
Fig.2 (□185.6nm) Co surface during CO blowing
Fig. 2 to Fig. 6 show sequential images obtained for the same surface with the same field of view when CO gas was blown onto the specimen at a pressure of 4.0 x 10-5 Torr in the chamber.
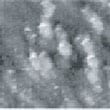
Fig.3 (□185.6nm) Co surface during CO blowing
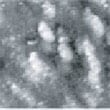
Fig.4 (□185.6nm) Co surface during CO blowing
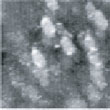
Fig.5 (□185.6nm) Co surface during CO blowing
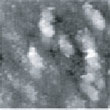
Fig.6 (□185.6nm) Co surface during CO blowing
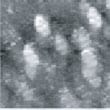
Fig.7 (□185.6nm) Co surface after CO blowing
Fig. 7 and 8 show images obtained after blowing was stopped.
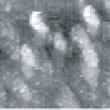
Fig.8 (□185.6nm) Co surface after CO blowing
It is possible that the island-shaped particle clusters grew while CO gas was blown onto the specimen in this way because CO gas was adsorbed by the cobalt surface.
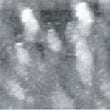
Fig.9 (□185.6nm) Co surface during CO blowing
Fig. 9 to Fig. 12 show sequential images obtained with the same field of view when CO gas was blown onto the specimen with various flow rates (at a pressure of 1.0 x 10-4 Torr in the chamber). In Fig. 9 and 10, the island-shaped particle clusters have grown even bigger. In Fig. 11 and 12, it can be seen that the surface morphology has changed significantly.
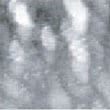
Fig.10 (□185.6nm) Co surface during CO blowing

Fig.11 (□185.6nm) Co surface during CO blowing

Fig.12 (□185.6nm) Co surface during CO blowing
Possible explanations as to why the surface morphology changed so much when CO gas was blown onto the specimen include the following. It is possible that cobalt carbonyl complex was generated, it moved away and became detached from the surface, and the surface morphology changed as a result. In this scenario, cobalt carbonyl complex is generated by the following reaction:
nCo + mCO → Con(CO)m↑
(Normally, n = 2 and m = 8.)
Alternatively, it is also possible that CO gas was adsorbed by the cobalt surface, causing the surface morphology to change, and that the changed surface became apparent when the CO moved away and became detached from the surface.
Because the changes in the surface morphology that result when CO gas is blown onto cobalt surface overall occur more slowly than the corresponding changes for the nickel surface described in the previous document, we can conclude that the reactivity between cobalt and CO gas is inferior to that between nickel and CO gas.
Real Time Observation of Pt Surface Morphology Changes due to CO Blowing
With the platinum surface, H2 gas was blown onto it to remove any oxygen present at the surface and, maintaining the surface in a clean state, CO gas was blown onto it.

Fig.13 (□185.6nm) Pt surface before CO blowing
Fig. 13 shows the image obtained by STM observation of the surface after reduction processing was performed using H2 gas and before CO gas was blown onto it. Fig. 14 shows the image obtained with the same field of view when CO gas was blown onto the surface at a pressure of 6.0 x 10-5 Torr in the chamber. Fig. 15 shows the image obtained after the blowing of CO gas was stopped.
In Fig. 13, which shows the surface before CO gas was blown onto it, island-shaped particle clusters of metal thin film can be observed. In Fig. 14, it can be seen that these particle clusters have grown.
The island-shaped particle clusters grew when CO gas was blown onto the surface probably because CO was adsorbed almost uniformly by the platinum surface.
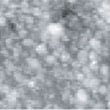
Fig.14 (□185.6nm) Pt surface during CO blowing

Fig.15 (□185.6nm) Pt surface after CO blowing

Fig.16 (□185.6nm) Pt surface before CO blowing
Fig. 16 shows the image obtained with the same field of view when CO gas was blown onto the surface with higher flow rates (at a pressure of 1.0 x 10-4 Torr in the chamber).

Fig.17 (□185.6nm) Pt surface after CO blowing
Fig. 17 shows the image obtained after the blowing of CO gas was stopped.
It can be seen from these images that, after CO has been uniformly adsorbed, the surface morphology hardly changes at all even when CO gas is blown onto it at higher flow rates.
As a result of observing the changes in surface morphology that occur when CO gas is blown onto nickel, cobalt, and platinum specimens, it can be concluded that the degree of change in surface morphology resulting from the reaction with CO gas can be characterized in the following way:
Ni > CO >> Pt
It can also be concluded that, while nickel consists of parts that adsorb CO readily and parts that do not, cobalt and platinum adsorb CO almost uniformly.
Here, we successfully used an STM in a gas atmosphere (Shimadzu WET-901 Environment-controlled STM) to successfully perform direct, continuous, real-time observation of the macro-level changes in surface morphology caused by the reaction that occurs when polycrystalline metal thin films come in contact with CO. The fact that it is now possible to perform surface observation using specimens and atmospheric conditions that are close to the actual reaction system means that the range of applications in which Environment-controlled STMs are used as tools to investigate the basic mechanism of changes in the surface morphology of materials can be expected to broaden.
The specimens used in this experiment were provided by the National Institute of Materials and Chemical Research.


