Core principles
MS is a very useful and powerful tool in quantitative and qualitative analysis. Furthermore, it can identify analytes based on m/z and provide accurate mass information on elemental composition, isotopic analysis and structural information. However, there are limitations of a single mass spectrometer. A single MS may not provide reliable quantitative and qualitative information in cases where resolution is insufficient (hard to separate) for both chromatography and m/z (e.g. isomers). This is particularly the case where the sample matrix is complex and the target analytes are in trace concentrations. Therefore, a technique that provides a higher selectivity, specificity and sensitivity and gives additional unique mass and structural information of the target analytes is required.
A MS/MS system, also known as a tandem/hybrid*6 MS (denoted MS/MS), consists of two mass analyzers connected in series with a collision or fragmentation cell in between. Ions are separated in the first mass analyzer (MS1), enter the collision cell and undergo fragmentation, resulting in generation of ions called product ions which are separated in the second mass analyzer (MS2) and detected. MS/MS serves as a solution for the challenges faced by a single MS analysis. Since its introduction, MS/MS has been a versatile and powerful instrument for many applications such as (1) drug discovery and development, (2) structural analysis of polysaccharides and proteins and (3) metabolomics identification and quantitation research. Notably, the use of MS/MS provides detailed mass information and allows the reduction of matrix interferences and background giving superior selectivity and sensitivity.
The basic components in a MS/MS system are illustrated in Figure 19. Like LCMS, MS/MS system can be coupled to a LC or GC for chromatographic separation prior to MS analysis. The main difference between a LCMS and a LC-MS/MS is the addition of a collision cell and a MS2. The single mass analyzers described earlier (e.g. quadrupole, TOF, ion trap or magnetic sector MS) can be integrated into a MS/MS system.
*6 Tandem MS refers to MS/MS systems that use the same mass analyzers (e.g. triple Quadrupole MS/MS) while hybrid MS refers to MS/MS systems with different MS systems (e.g. Quadrupole-TOF MS).

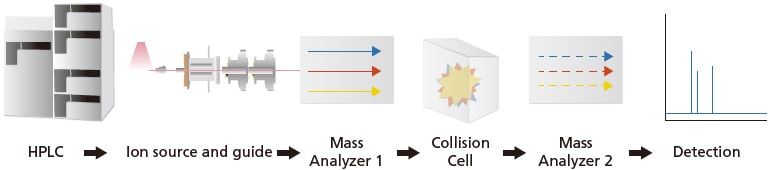
Figure 19. Basic instrumentation of a LC-MS/MS.
Collision-induced dissociation (CID) is the preferred method of fragmentation in the collision cell. Fragmentation of the precursor ions occurs and this provides unique MS data of the fragment ions. Figure 20 illustrates the processes and fragmentations that occurs in the collision cell.
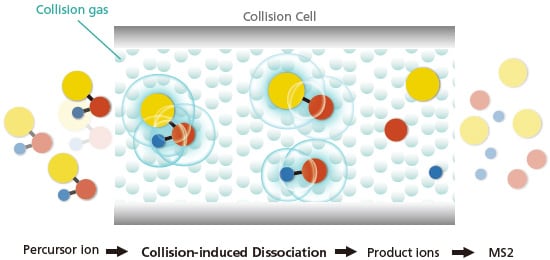
Figure 20. Illustration of CID in the collision cell of MS/MS systems.
The precursor ions of m/z selected by MS1 enter the collision cell filled with chemically inert gas (e.g. He, Ar, Xe and N2) and collisions between the precursor ions and inert gas are induced by applying an oscillatory field (giving a "shake"). Collisions cause conversion of kinetic energy into molecular excitation (internal energy) that cause chemical bond breakage to generate product ions. The degree of fragmentation and product ion species depend on the energy supplied because some bonds require higher activation energy than others for breakage. As shown in Figure 21, with a CID collision energy of 0 volts, the molecular ion of Osteltamivir (m/z = 313) is of highest abundance with no product ions. As collision energy increases, the abundance of the molecular ion decreases and fragmentation occurs to generate a variety of product ions. At even higher collision energies (e.g. >50 V), the degree of fragmentation is more extensive resulting in the mass spectrum showing no molecular ion and higher abundances of product ions of lower m/z. Besides CID, there are other less common alternatives to induce fragmentation such as electron capture/transfer dissociation, surfaceinduced dissociation and photodissociation.
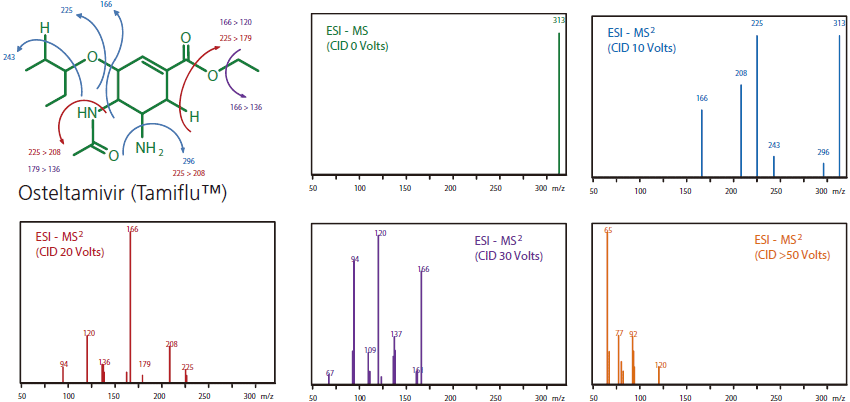
Figure 21. CID of Osteltamivir (Tamiflu) at various collision energies (0, 10, 20, 30, >50 V).
Figure 16 describes the scan and selected ion monitoring (SIM) modes of a quadrupole MS. Likewise in MS/MS, the MS1 and MS2 can either acquire the scan or SIM (fixed) mode. The different arrangements allow the system to operate at various scan and monitoring modes (Figure 22). It is important to note that the actual scan and monitoring modes available in MS/MS systems depends on the mass analyzers (MS1 and MS2) used. Some of these scan modes (e.g. precursor ion and neutral ion scan) may not be available in time-based*7 MS (e.g. ion trap and FT-ICR).
For a long time, Selected Reaction Monitoring (SRM) used to be the term historically preferred by IUPAC, while instrument vendors including Shimadzu preferred the alternative term, Multiple Reaction Monitoring (MRM). Now IUPAC recommends that the term SRM be used to describe the technique applied to a single target and MRM for plurality of SRM. In this document hereafter, the technique is referred to as MRM regardless of the number of precursor/product pairs monitored simultaneously.
Prior to performing MRM, product ion scan is usually conducted to determine the product ion of highest abundance. In conclusion, MS/MS can not only remove noise and interference but also selectively target particular ions for quantitation to deliver a higher specificity and sensitivity analysis.
*7 Time-based MS refers to ion trap and FT-ICR MS, where ions are separated temporally and gets detected sequentially at a fixed point. On the other hand, spacebased MS separates ions spatially based on their deviation in terms of path radius or velocity. Examples of space-based MS are magnetic sector, quadrupole and TOF MS.
(A) Product Ion Scan:
MS1 is fixed at selected m/z while MS2 operates at scan mode. This scan acquires all the product ions from the fragmentation of a selected precursor ion. The intensity and m/z of all product ions are captured and displayed in the mass spectrum.
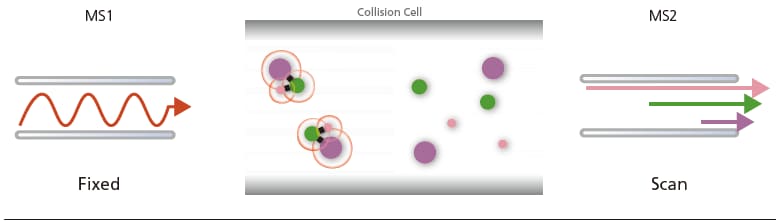
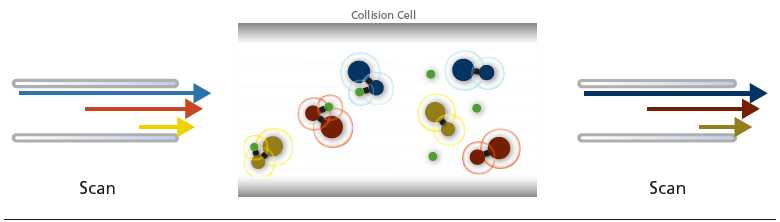
(B) Precursor Ion Scan:
MS1 operates at scan mode while MS2 selects the product ion of a particular m/z formed by CID in the collision cell. This mode is especially useful for determining the precursor ions that produce fragment ions of a particular m/z.
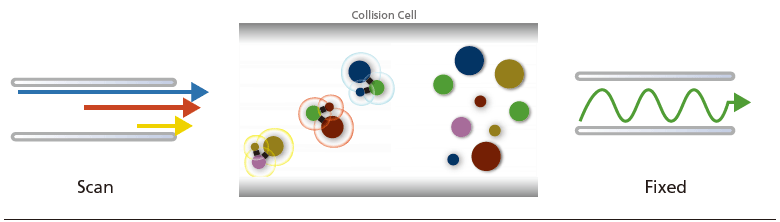
(C) Neutral Loss Scan:
MS1 and MS2 operates at scan mode while keeping a specific m/z difference. This scan can determine the precursor ions that lose a specific neutral molecule (e.g. hydroxyl group and phosphate group) during fragmentation.
(D) Selected Reaction Monitoring (SRM):
The transition of a selected precursor ion to a selected product ion is monitored where MS1 and MS2 are fixed at the specific m/z.
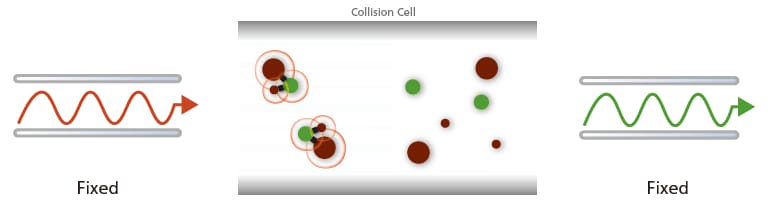
Figure 22. Types of scan and monitoring modes in MS/MS systems.



