The Horror of Sample Adsorption to Containers (Part 1)
Technical Advisor, Analytical & Measuring Instruments Division,
Shimadzu Corporation
There have been amazing advances in LC/MS system development and separation technology in recent years, along with the increasingly widespread use of LC/MS as an appealing analytical tool for various applications, including the elucidation of vital phenomena, disease diagnosis, pharmaceutical development, and fields related to food and the environment. Of these applications, due to its highly selective and highly sensitive detection performance, LC/MS is an essential analytical tool for the quantitation of trace sample components.
However, an intrinsic problem arises when using LC/MS for the quantitation of trace components. Because LC/MS is capable of detecting with high sensitivity, the samples used in LC/MS are very low in concentration. These low sample concentrations result in a situation where sample adsorption to containers and instruments can be a crucial factor in reducing the reliability of quantitative results. Those involved in analytical chemistry are in the business of "inquiry into how to ensure highly reliable analytical results are obtained both simply and quickly," but I wonder if many analysts consider this issue of adsorption to containers.
It is clear to most that LC/MS will play an important role in the future as a highly sensitive quantitative method. Consequently, increasing the reliability of highly sensitive quantitative analyses performed using LC/MS is of is of the utmost importance. In this issue, I focus on sample adsorption to containers, a problem often overlooked by many analysts, hoping to contribute towards improving analyses using LC/MS.
My writing for this issue brought back frustrating memories of sample adsorption to containers. As far back as 35 years ago, HPLC was used to analyze peptide formulations of around 20 residues. HPLC systems of the time were far removed from the automated and sophisticated HPLC systems available today, using a microsyringe (with ground glass internal surface) to obtain a fixed sample volume (of several g/mL) that was injected manually into an injector. Variability between results was substantial at the time regardless of how many times the analysis was repeated, and seeing such results were alarming. During the trial and error stage of investigating the cause of this variability, I noted how the more thoroughly a microsyringe was rinsed, and the longer a sample resided inside the syringe, the lower the detected peaks.
I eventually realized that variability was caused by adsorption of sample to the microsyringe. Realizing to my horror the great effect mere adsorption was having on the reliability of my results, I felt keenly the need to control adsorption during the sample manipulation process from sample preparation to HPLC injection. Contrasted against how the phenomenon of adsorption is mostly overlooked today with the automated HPLC systems used in recent years, such bitter experiences have increased my understanding of adsorption and become a valuable motive in my work towards controlling adsorption to containers and instruments. These experiences brought adsorption to containers and instruments to the forefront of my mind, and I now make a point of always approaching sample preparation by first understanding the phenomenon of adsorption to containers and instruments.
I recently heard an LC/MS analysts say, "target component peaks in plasma sample solutions are detected, but no peaks are detected in standard solution" or, "when I analyze a standard sample solution prepared the previous day, no peaks are detected." While the cause of these issues could be attributed to degradation based on sample stability, in the majority of cases it is easily recognized as container adsorption. Particularly with plasma samples, we know that impurities in the sample adsorb preferentially to containers, preventing the adsorption of target components. This is the same thinking behind using bovine serum albumin (BSA) as an adsorption prevention agent. In any event, as long analytical evaluation by LC/MS continues to play an important role and carry the weight of our expectations, we are called on to attempt to understand thoroughly the phenomenon of sample adsorption to containers in order to procure highly reliable results.
In this series of experiments, I have interpreted the mechanism of sample adsorption to containers in terms of the mechanism of HPLC separation. The mechanism of separation during HPLC amounts to no more than using a mobile phase to control the strength of adsorption of a solute on a stationary phase. When this mechanism is translated to the phenomenon of sample adsorption to containers, the stationary phase becomes the container material, the mobile phase becomes the sample solution being prepared, and the retention behavior of materials adsorbed onto containers is the adsorption strength.
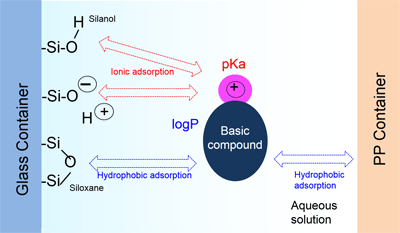
Fig. 1 Mechanism of Adsorption to Vials and Methods for Inhibiting Adsorption
Containers used in sample preparation normally come in one of two main materials: glass containers and plastic containers (polypropylene: PP, polyethylene: PE). The surface of glass containers is covered in highly hydrophilic silanol and hydrophobic siloxane groups (dehydrated silanol, which arises during melting/formation of silica gel at high temperatures). Consequently, in an aqueous solution, glass containers simultaneously elicit ionic adsorption of molecules to silanol groups (positive ion exchange mode) and hydrophobic adsorption due to siloxane groups (reverse phase). This phenomenon of ionic adsorption is analogous to the phenomenon of peak tailing and increased retention of basic compounds caused by residual silanol in an HPLC column. For plastic containers, the only adsorption caused by the container material (high molecular weight polymers) is hydrophobic adsorption. That is, the mechanism of adsorption differs depending on the container material. (See Fig. 1.) Because of these characteristics, basic compounds with a high pKa (acid dissociation constant) tend to adsorb to glass (silanol), and compounds with a large logP (octanol/water partition coefficient) tend to adsorb to both glass containers and plastic containers. During sample preparation, one of the measures taken to prevent adsorption is to first select containers based on the physical properties of the target component (compound pKa and logP).
Looking back on these frustrating experiences, since adsorption to containers is more apparent with low-concentration samples, finding conditions for low-concentration sample preparation that reduce adsorption to containers and instruments is very important for ensuring reliability of analytical methods.
Below I have described my method of sample preparation that reduces adsorption to containers. The first thing to confirm whether the target compound/s are basic, acid or neutral.
Next, different sample preparation methods should be used depending on whether sample preparation will performed in glass containers or PP containers.
1) Acidic compounds and neutral compounds
Acidic compounds and neutral compounds mainly adsorb to glass and PP containers by hydrophobic adsorption. Therefore, an organic solvent (methanol, acetonitrile, etc.) or non-ionic surfactant is added to the sample solution to reduce adsorption. Although dependent on the logP of the target compounds, adding 10 to 50 % organic solvent or around 0.1 % surfactant is normally effective for reducing adsorption during sample preparation.
2) Basic compounds
Care should be taken when selecting container materials for sample preparation, as basic compounds adsorb to glass containers and PP containers by different mechanisms. As an example, an outline of the method used to reduce adsorption of basic compounds is shown in Fig. 2.
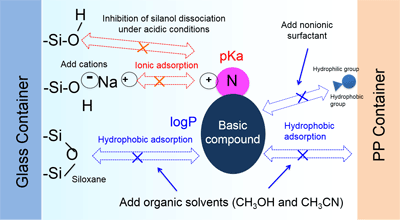
Fig.2 Mechanism of Adsorption to Vials and Methods for Inhibiting Adsorption
i) Glass containers
Basic compounds adsorb to glass containers by both ionic adsorption and hydrophobic adsorption simultaneously, so sample preparation must be adjusted to counter both mechanisms of adsorption. Salt (NaCl, etc.) is added to place positive salt ions (Na+, etc.) in solution that reduce ionic adsorption by blocking interaction with silanol groups. Alternatively, silanol dissociation can be prevented by performing sample preparation under acidic conditions (by addition of phosphoric acid, acetic acid, etc.). As with acidic and neutral compounds, hydrophobic adsorption is prevented by adding organic solvent or non-ionic surfactant to the sample solution.
ii) PP containers
PP containers give rise to hydrophobic adsorption, so to reduce this adsorption an organic solvent or non-ionic surfactant is added to the sample solution.
An effective means of reducing adsorption in both glass and PP containers is therefore to have both a salt and either an organic solvent or non-ionic surfactant present in the sample solution.
When performing a highly sensitive quantitative analysis using HPLC or LC/MS, consideration must also be given to the effect on separation of sample adjustments made to reduce adsorption, and the resulting effect on ion suppression during MS. Furthermore, analytical columns with smaller diameter packing and internal diameters are becoming widespread since they improve HPLC and LC/MS performance, and these changes must also be considered alongside how the quantity of organic solvent in the sample solution (injected solution) affects separation. The reality of highly sensitive quantitative analysis is that many investigations are required in order to determine optimum sample solution conditions for reducing adsorption to containers.
I have focused on the development of versatile low-adsorption vials suitable for use in HPLC auto samplers, where the surface finish of the container is optimized to reduce ionic and hydrophobic sample adsorption.
In the next issue of this journal, I will discuss how specially-developed vials reduce adsorption, using peptides and basic compounds as specific example compounds.
>>The Horror of Sample Adsorption to Containers Part 2


