Raw Materials Identification Testing by NIR Spectroscopy and Raman Spectroscopy
This FTIR TALK LETTER compares NIR spectroscopy and Raman spectroscopy while introducing their respective features and some examples of sample measurements. These methods make analysis easy and are attracting attention for acceptance testing on all raw materials as required by PIC/S GMP guidelines, verification and identification of received raw materials, intermediate and final product inspections, and counterfeit drug detection. The PIC/S GMP guidelines are outlined below.
1. PIC/S GMP Guidelines
International trends in the quality assurance of drugs have changed dramatically in recent years. There is now a demand for overseas quality assurance measures to also be implemented in Japan. The increased need for international cooperation and information exchange with regard to GMP*1 brings a demand for more advanced business implementation systems.
Under these circumstances, Japan's Ministry of Health,Labour and Welfare applied for membership of PIC/S*2 in March 2012. As future GMP inspections will be based on the PIC/S GMP guidelines, analytical laboratories must comply with them as rapidly as possible.
The PIC/S GMP guidelines require acceptance testing on all raw materials. NIR spectroscopy and Raman spectroscopy, are attracting attention as inspection methods suitable for efficient on-site identification testing. Currently, the Japanese Pharmacopoeia (JP) prescribes NIR spectroscopy. On the other hand, the US Pharmacopoeia (USP) and European Pharmacopoeia (EP) prescribe Raman spectroscopy. Almost all major pharmaceutical corporations in countries other than Japan use Raman spectroscopy for the identification of received raw materials.
*1 GMP
This is the acronym of Good Manufacturing Practice. GMP offers manufacturing control and quality control guidelines for drugs and quasi drugs. It is a standard enacted by government and other public organizations to ensure safety and reliability. The GMP Ministerial Ordinance prescribes validation standards related to the manufacture of drugs and quasi drugs and the conditions to be observed by manufacturers.
*2 PIC/S
This is the acronym of Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme. As of January 2013, 41 countries were members, predominantly European countries. PIC/S is intended to provide an internationally harmonized standard and devise a quality assurance system for the continuous implementation of GMP compliance for pharmaceuticals. The PIC/S GMP Guidelines have been published as part of these activities.
2. Comparison of NIR Spectroscopy and Raman Spectroscopy
As near-infrared (NIR) spectroscopy and Raman spectroscopy obtain spectra based on molecular vibrations, they both permit qualitative analysis.
The term "near infrared" indicates light with wavelengths between 800nm and 2500nm (12500 to 4000cm-1 wavenumber). Absorption appearing in the near-infrared region involves overlaid harmonic and combination tones from normal vibrations and therefore produces wider and more complex peaks than does absorption in the mid-infrared region. Also, since the absorption intensity is lower than in the mid-infrared region, samples can be measured directly without being diluted with KBr or other agents.
Furthermore, cells made of glass or quartz, which are chemically stable and easy to use, can be used for NIR spectroscopy because they exhibit virtually no absorption in the near-infrared region. For details on NIR spectroscopy, refer to "Near-Infrared Region Measurement and Related Considerations" in FTIR TALK LETTER Vol. 9 and Vol. 10.
In this example of near-infrared analysis, we used the IntegratIR NIR integrating sphere shown in Fig. 1. Table 1 shows the measurement conditions. All sample types, including powder, pill, liquid, and paste samples, can be placed on the stage for measurement. Samples can also be measured when contained in a plastic bag or glass bottle.

Fig. 1 IntegratIR Integrating Sphere
Table 1 Instrument and Analytical Conditions
| Instrument: | IRPrestige-21, IntegratIR |
| Resolution: | 16.0cm-1 |
| Accumulation: | 20 |
| Apodization: | Sqr Triangle |
| Light Source: | Tungsten lump |
| Beam Splitter: | CaF2 |
| Detector: | InGaAs |
Meanwhile, Raman spectroscopy uses visible or near-infrared laser light. It measures the light scattered from the sample when it is illuminated with light of a certain wavelength. Raman spectra are plotted with the scattering intensity on the vertical axis and the wavenumber difference between the incident light and scattered light (the so-called Raman shift) on the horizontal axis. The horizontal axis units are cm-1, the same as for IR spectra. In this example, we used an excitation wavelength of 785 nm and a resolution of 6.0 cm-1 as measurement conditions. Raman spectroscopy can measure samples contained in a plastic bag or glass container that allows light to pass through it, without the need to unseal the sample. However, measurement may not be possible with certain container materials and material thicknesses.
For details on the differences between Raman spectroscopy and infrared spectroscopy, refer to Q&A in FTIR TALK LETTER Vol. 17 as well.
Table 2 summarizes the features of NIR spectroscopy and Raman spectroscopy. So far, we have touched on items (1) and (2). Section 3 below covers items (3) to (6) and introduces measurements of actual samples.
Table 2 Comparison of NIR Spectroscopy and Raman Spectroscopy
| Item | NIR Spectroscopy | Raman Spectroscopy |
| (1) Pharmacopoeia support | Japanese Pharmacopoeia (JP), US Pharmacopoeia (USP),European Pharmacopoeia (EP) | US Pharmacopoeia (USP),European Pharmacopoeia (EP) |
| (2) Light used | Near-infrared light | Visible or near-infrared laser light(Requires measures related to using laser light.) |
| (3) Spectral shapes | For samples with similar structures,wide peaks make differences hard to see. | For samples with similar structures,sharp peaks clearly show differences. |
| (4) Samples unsuited to measurement | Samples with weak NIR absorption(inorganic compounds etc.) | Samples with weak Raman scatteringSamples susceptible to effects of fluorescenceSamples that decompose or burn due to visible or NIR laser light |
| (5) Effects of particle size | Separate standard data registrationrequired for different particle sizes | None |
| (6) Effects of container | Separate standard data registration required foreach container type and thickness. | A container transparent to visible orNIR laser light has little effect. |
3. Examples of Sample Measurement by NIR Spectroscopy and Raman Spectroscopy
1) Spectral Shapes and Samples Unsuitable for Measurement (Table 2 (3)(4))
We analyzed titanium dioxide anatase (TiO2 anatase) and calcium carbonate (CaCO3) as examples of inorganic compounds. The measured results are shown in Fig. 2. Inorganic compounds exhibit weak absorption in the near-infrared region and produce wide peaks. Be aware that, while not shown here, hydroxyl-group-derived peaks may appear in the spectrum when the sample has absorbed moisture.
On the other hand, Raman spectroscopy produces sharp peaks, even for inorganic compounds. Another feature of Raman spectroscopy is that hydroxyl-group-derived peaks are not easily detected.
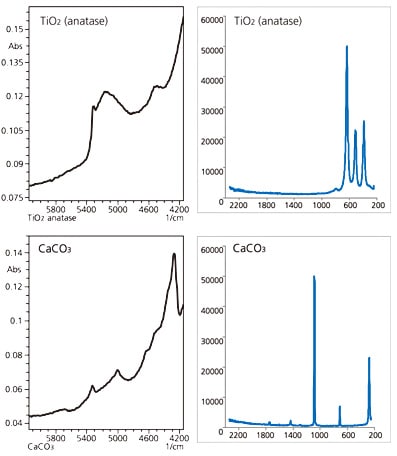
Fig. 2 Analysis of Inorganic Compounds(TiO2 anatase and CaCO3)Left: NIR Spectroscopy; Right: Raman Spectroscopy
Next, we measured lactate monohydrate and microcrystalline cellulose as examples of organic compounds. The measured results are shown in Fig. 3.
Comparatively strong NIR absorption was detected for the organic compounds but the broad peaks and varying shapes made evaluation difficult. On the other hand, Raman spectroscopy produced sharp peaks but may result in an ascending baseline for fluorescent samples, such as lactate monohydrate. Care is required with the evaluation of fluorescent samples with low peak intensity and few peaks, such as microcrystalline cellulose.
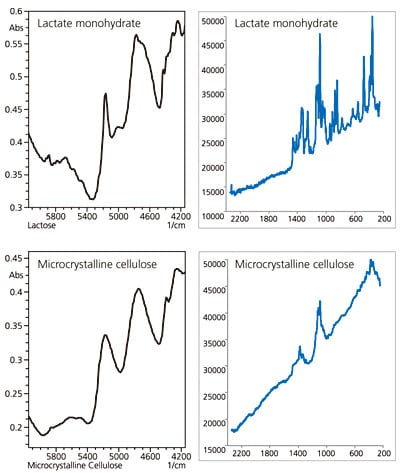
Fig. 3 Analysis of Organic Compounds
(Lactate Monohydrate and Microcrystalline Cellulose)
Left: NIR Spectroscopy; Right: Raman Spectroscopy
2) Effects of Particle Size (Table 2 (5))
To investigate the effects of particle size, we measured stearic acid with different particle sizes. The measured results are shown in Fig. 4. With NIR spectroscopy, the characteristic changes in spectral shape due to the effects of physical properties can be used not only for qualitative analysis but also to obtain information on particle size. NIR spectroscopy is therefore used to monitor all stages of pill manufacture (raw materials acceptance, pulverization, mixing, granulation, drying, tableting, and coating). In this example of stearic acid measurements, Raman spectroscopy, which produces clear peaks, is more effective for component identification than NIR spectroscopy. However, NIR spectroscopy works better to determine differences in particle size.
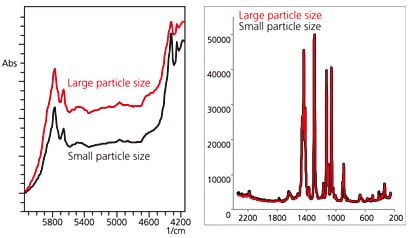
Fig. 4 Effects of Particle Size (Stearic Acid)
Left: NIR Spectroscopy; Right: Raman Spectroscopy
3) Effects of Containers (Table 2 (6))
We next measured talc [Mg3(Si4O10)(OH)2] sealed inside polyethylene (PE), polypropylene (PP) and polyethylene terephthalate (PET) plastic bags. The NIR spectroscopy measurement results are shown in Fig. 5, where the spectral shape varies according to the container material. For this reason, to perform acceptance testing of raw materials using NIR spectroscopy, standard data for pass/fail criteria must be registered for each container material. Also, interference fringes can be seen in the NIR spectrum for the PP bag. These may appear due to the condition and thickness of the bag and how the sample is packed, and may impair peak identification.
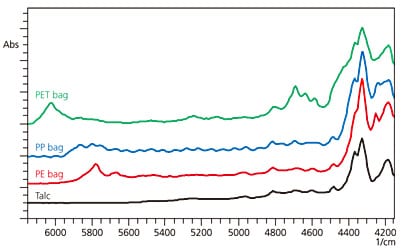
Fig. 5 Effects of Containers on NIR Spectroscopy
(Talc in PE, PP, and PET Bags)
We subsequently analyzed lactose contained in polyethylene (PE) bags of different thicknesses. The measurement results are shown in Fig. 6. In the NIR spectroscopy results, peaks derived from PE are observed and the spectral shape changes according to the container thickness as well. Raman spectroscopy, on the other hand, is hardly affected by the material or thickness of the container, provided that the material is transparent to visible or near-infrared laser light.
Table 3 shows the suitability of different containers to NIR spectroscopy and Raman spectroscopy measurements.
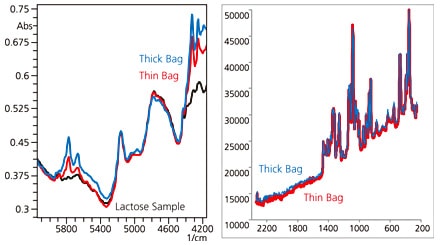
Fig. 6 Effects of Containers
(Thick Bag/Thin Bag, Lactose Sample)
Left: NIR Spectroscopy; Right: Raman Spectroscopy
Table 3 Suitability of Containers to Each Measurement
| Item | NIR Spectroscopy | Raman Spectroscopy |
| Glass bottle (colorless) | Good | Good *3 |
| Glass bottle (brown) | Good | Good *3 |
| Plastic bag | Good | Good |
| Plastic container | Fair | Good |
| Paper container | Poor | Poor |
| Metal | Poor | Poor |
*3 Some components contained in the glass may prohibit measurements.
4. Conclusions
We measured various samples by NIR spectroscopy and Raman spectroscopy to determine the pros and cons of each method.
Acceptance testing by NIR spectroscopy involves selecting peaks from a smooth spectrum and basing the evaluation on very small differences between them, which makes method creation difficult when an instrument is introduced. Although NIR spectroscopy offers some advantages, such as determining differences in particle size, Raman spectroscopy often makes it easier to evaluate the spectra. However, it is unable to measure samples that fluoresce under laser light. Take these factors into account when selecting the most suitable inspection method for actual samples.
References
- Administrative Circular "Practical Application of the PIC/S GMP Guidelines," issued by the Ministry of Health, Labour and Welfare, Feb. 1, 2012
- NIR and Raman Spectroscopy, edited by the Spectroscopical Society of Japan and published by Kodansha






