Ionization Modes: PCI
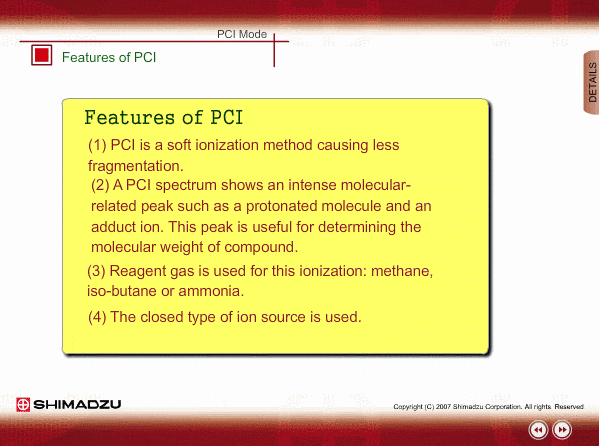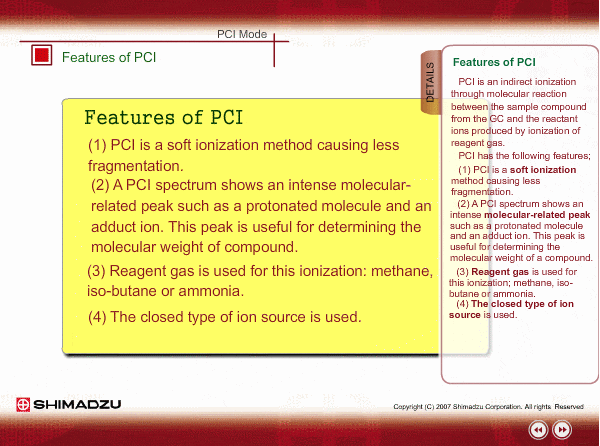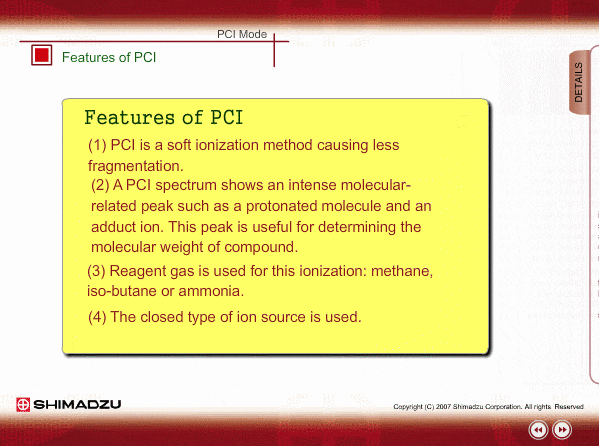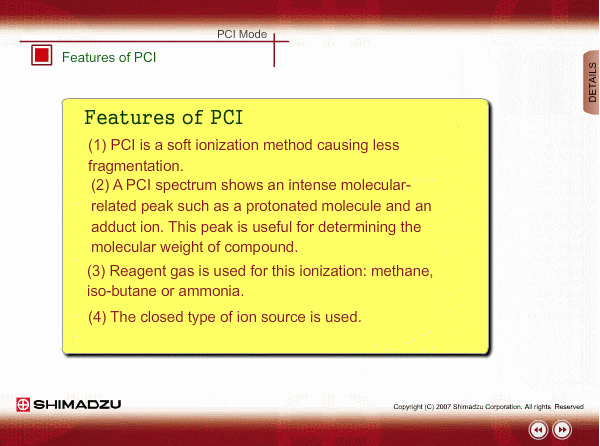
Features of PCI
PCI is an indirect ionization through molecular reaction between the sample compound from the GC and the reactant ions produced by ionization of reagent gas. PCI has the following features:
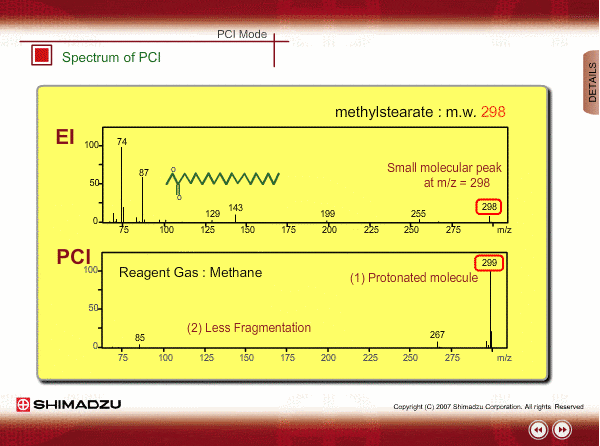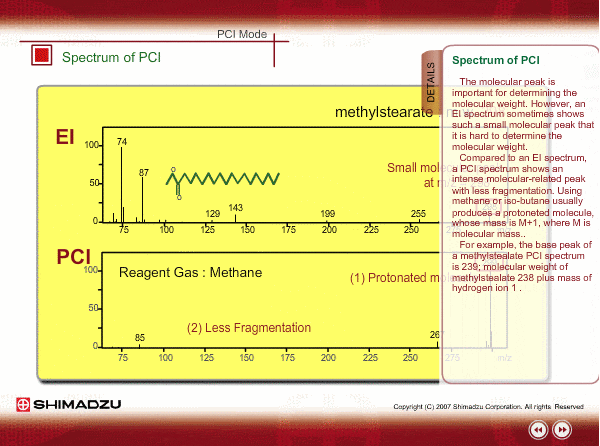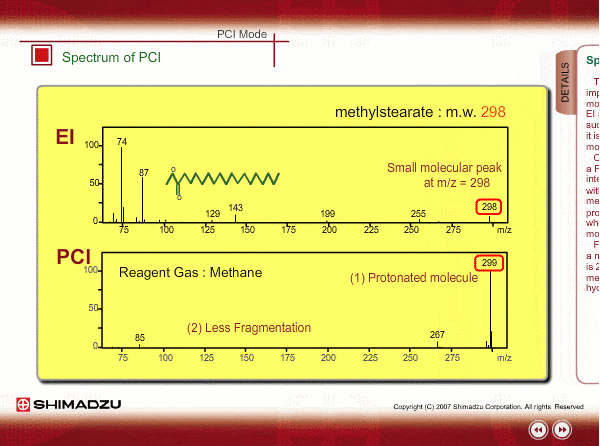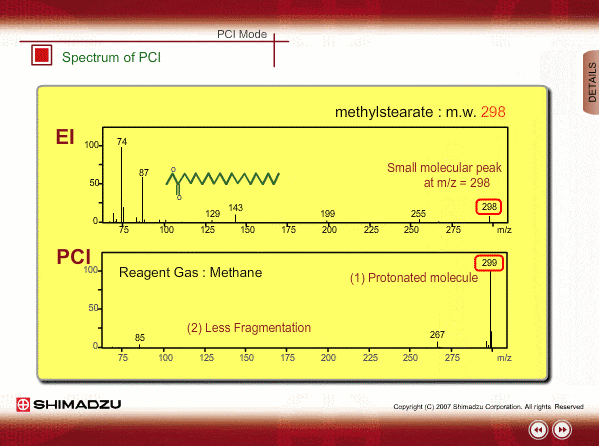
Spectrum of PCI
The molecular peak is important for determining the molecular weight. However, an EI spectrum sometimes shows such a small molecular peak that it is hard to determine the molecular weight. Compared to an EI spectrum, a PCI spectrum shows an intense molecular-related peak with less fragmentation. Using methane or iso-butane usually produces a protoneted molecule, whose mass is M+1, where M is molecular mass. For example, the base peak of a methylstealate PCI spectrum is 239; molecular weight of methylstealate 238 plus mass of hydrogen ion 1.
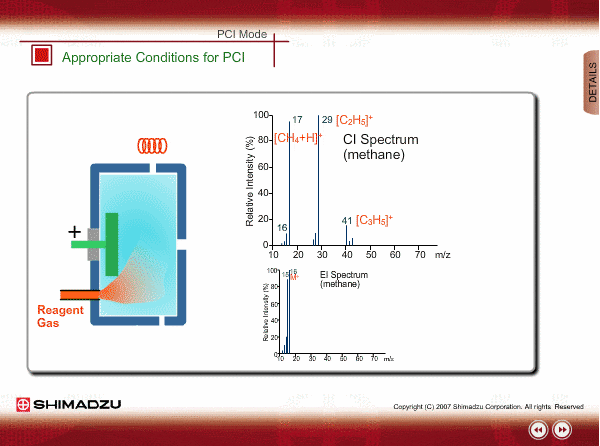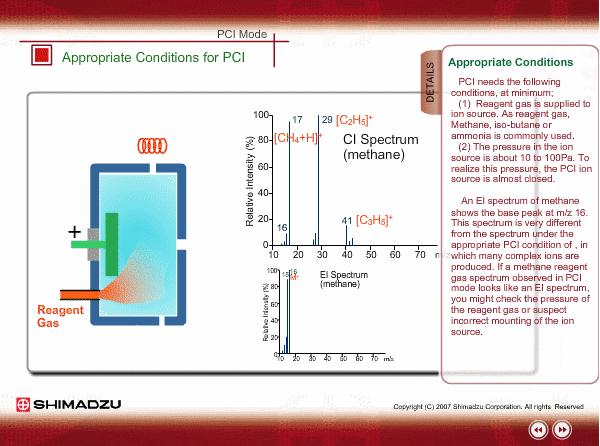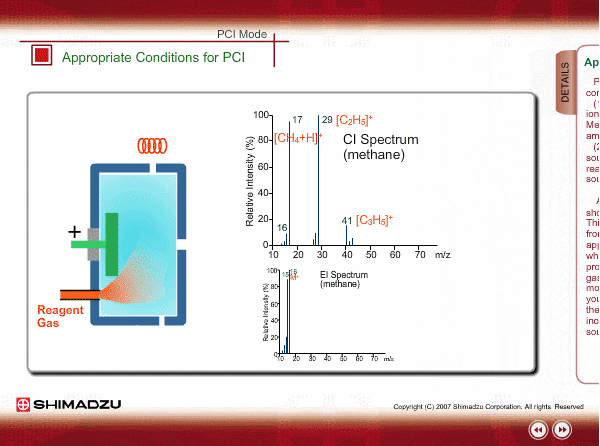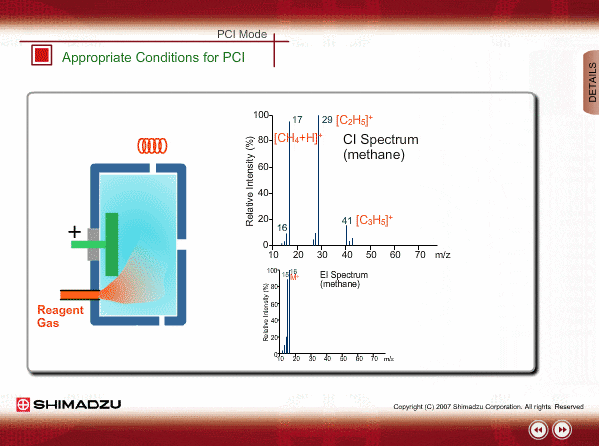
Appropriate Conditions of PCI
PCI needs the following conditions, at the minimum:
- Reagent gas is supplied to ion source. As reagent gas, Methane, iso-butane or ammonia is commonly used.
- The pressure in the ion source is about 10 to 100Pa. To realize this pressure, the PCI ion source is almost closed up.
An EI spectrum of methane shows the base peak at m/z 16. This spectrum is very different from the spectrum under the appropriate PCI conditions, in which many complex ions are produced. If a methane reagent gas spectrum observed in PCI mode looks like an EI spectrum, you might check the pressure of the reagent gas or suspect incorrect mounting of the ion source.
Ionization Process of PCI
The ionization process of PCI consists of these three steps:
- Reagent gas is ionized by EI.
- Reactant ions are created by reaction between ions produced in (1) and reagent gas molecules.
- Sample molecules are ionized by reaction with reactant ions.
Reactions Ionizing Sample Molecules
In PCI, samples are ionized by reaction between sample molecules and reactant ions in several ways. Some important reactions are summarized here.

Proton Affinity
The proton transfer is the most important reaction in PCI, because the peak of a protonated molecule, which is produced by this reaction, is indispensable in determining the molecular weight. The tendency of a sample molecule to accept a proton is measured by proton affinity P.A.

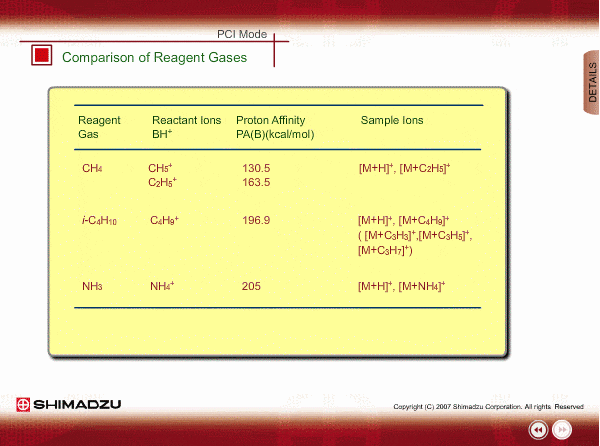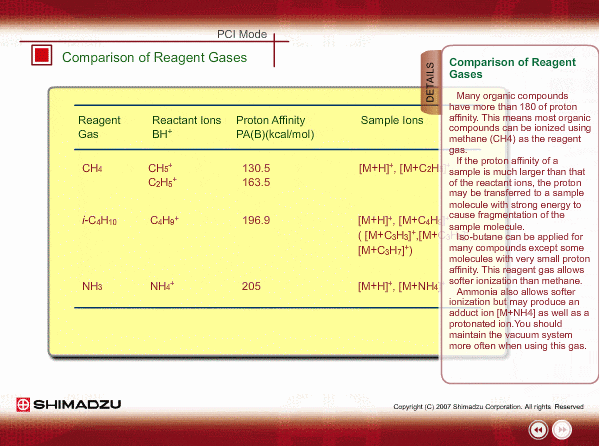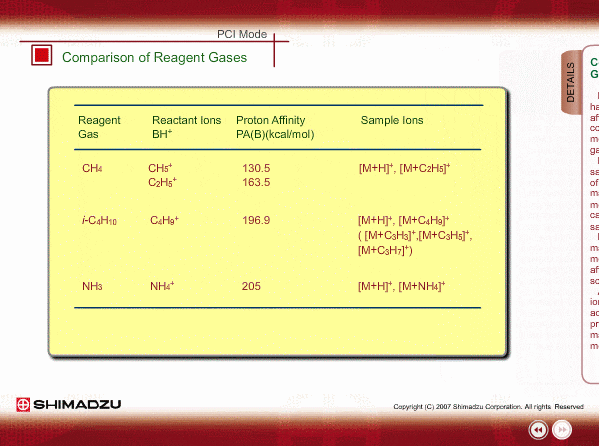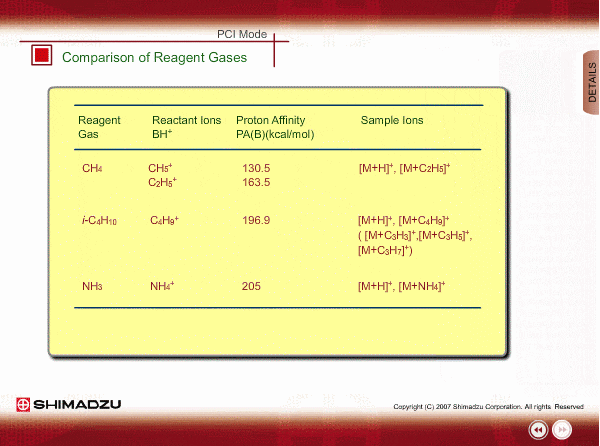
Comparison of Reagent Gasses
Many organic compounds have more than 180 of proton affinity. This means most organic compounds can be ionized as long as methane (CH4) is used as the reagent gas. If the proton affinity of a sample is much larger than that of the reactant ions, the proton may be fiercely transferred to a sample molecule with strong energy to cause fragmentation of the sample molecule.
Iso-butane can be applied for many compounds except some molecules with very small proton affinity. This reagent gas allows softer ionization than methane. Ammonia also allows softer ionization, but may produce an adduct ion [M+NH4] as well as a protonated ion. You should maintain the vacuum system more often when using this gas.