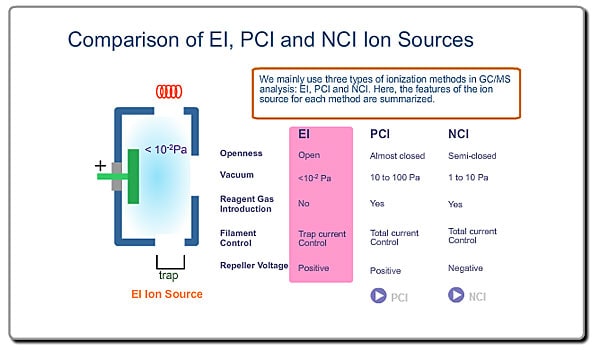
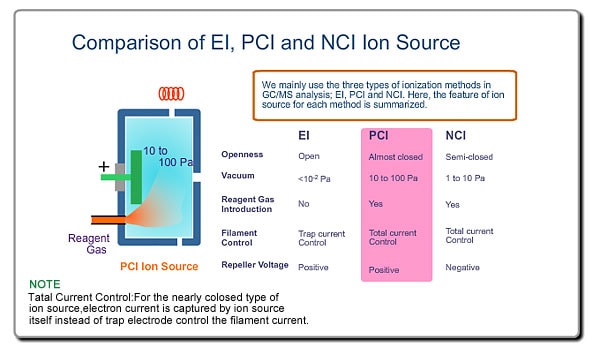
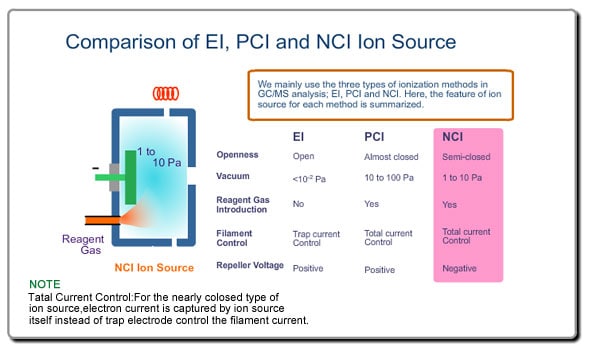
Ion Source
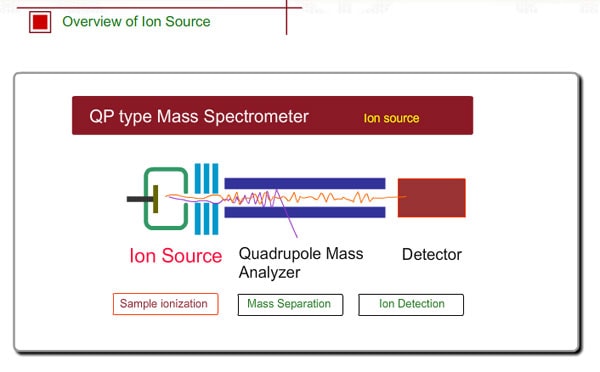
Overview of Ion Source
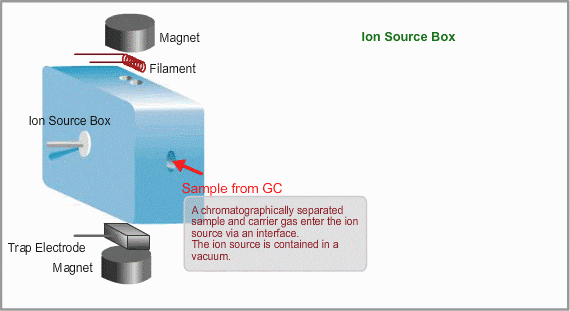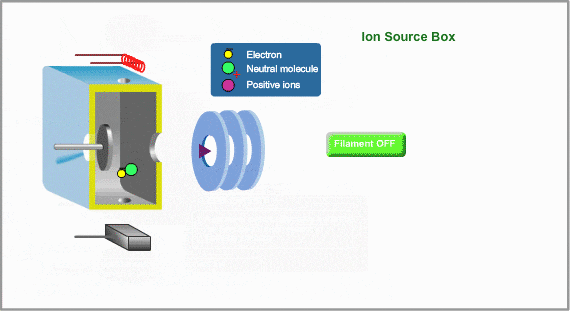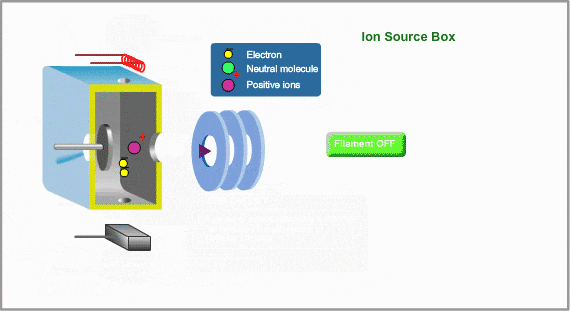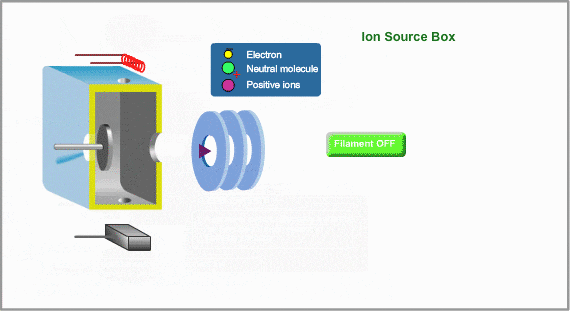
A Mass Spectrometer is an instrument which electrically analyzes the mass of a sample molecule. Therefore, molecules need to be ionized. A sample flowing from a GC is ionized in the ion source.
There are various ionization methods. Explanation of the ion source here will be based on the most typical ionization method, EI.
Structure of Ion Source
This is a typical ion source for EI (Electron Ionization). EI is the most common ionization method used in GC/MS Ions are produced in the ion source. Neutral molecules are hit by the electrons, emitted from the heated filament, to be ionized. Produced ions are electrically pushed out from the ion source by the positive voltage on the repeller electrode.
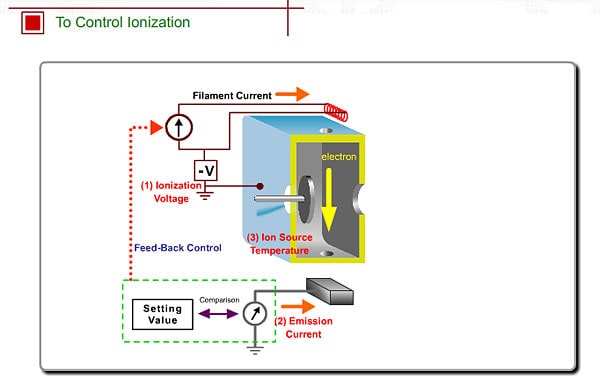
To Control Ionization
- Ionization Voltage Typically, 70eV electrons from the filament are used for EI. This energy is determined by voltage deference between the filament and the ion source. This voltage is changeable in some commercial GC/MS instruments
- Emission Current The number of ions produced in unit time is proportional to the current of emitted electrons, i.e., emission current. To stabilize formation of ions, the monitored emission current is always compared to the setting value and the result is feed-backed to the filament current source.
- Ion Source Temperature The ion source is usually used at about 200 oC.
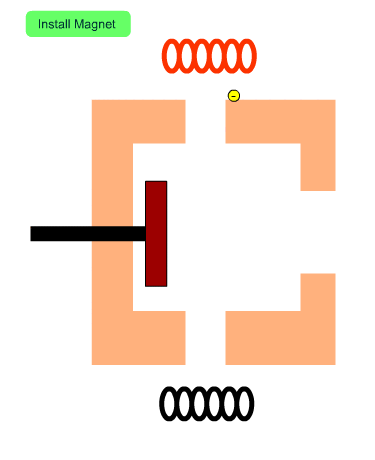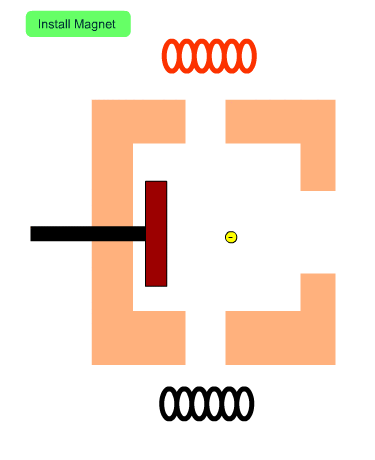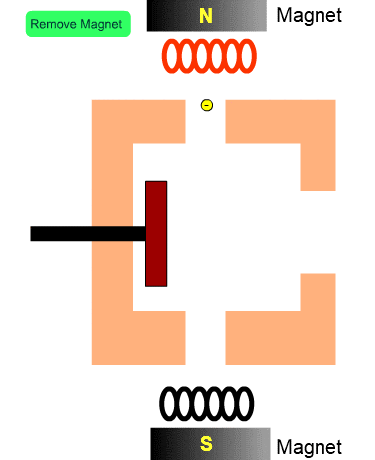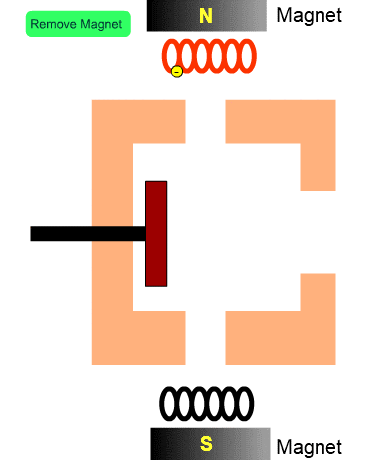
The Role of Magnets
The figure shows the cross section of the ion source with no magnets. Electrons from the filament tend to diverge. When a magnetic field exists, electrons will fly along the magnetic flux. In other words, magnets installed in the ion source increase the number of electrons entering into the ion source and enhance the efficiency of ionization.