7 Key Differences in the Use of Methanol and Acetonitrile
Methanol and acetonitrile are organic acids commonly used as the mobile phase in reverse-phase chromatography. The properties of these two organic acids differ, and below we outline 7 key differences to keep in mind when using methanol or acetonitrile for analysis.
1.Column Pressure
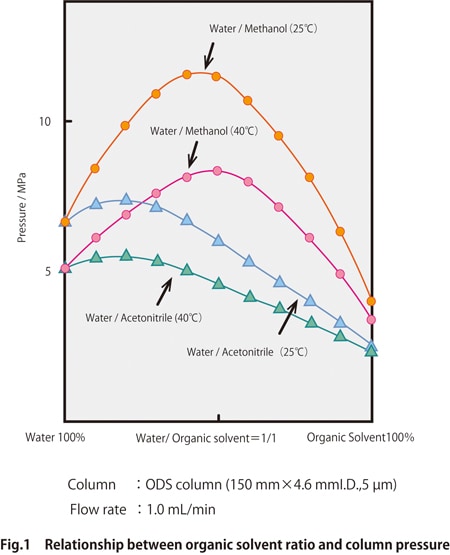
Fig. 1 shows an example of the solvent ratio and solvent delivery pressure for mixtures of water/acetonitrile and water/methanol.
The pressure also tends to become lower as the viscosity of the solvent decreases due to the higher column temperature. Setting the column temperature between 25-40 oC and comparing the column pressures for water/acetonitrile and water/methanol, we can see that the pressure is higher for methanol. When switching the mobile phase from acetonitrile to methanol, the pressure resistance of the equipment and column should be rechecked.
2.Absorption Spectrum
Figs. 2 and 3 show the absorption spectra of acetonitrile and methanol, including both commercial-grade solvent for HPLC use and high-grade solvent. Commercial organic solvents for HPLC have been processed to remove virtually all impurities and to display absorbance within set limits between specified wavelengths. It can be seen from Fig. 2 that the absorbance of HPLC-grade acetonitrile is particularly low at short wavelengths. This HPLC-grade acetonitrile is therefore suited to high-sensitivity analysis with UV detection in the short-wavelength region. Moreover, organic solvents which have been processed for LCMS analysis have both UV-absorbent impurities and residual metals removed. This acts to prevent background noise specific to LCMS analysis. When changing the organic solvent from acetonitrile to methanol, ghost peaks may be detected in gradient analysis due to the analytical conditions in the UV short wavelength range. In this case, we recommend reconsidering the solvent grade. If the cause of the ghost peaks is unclear and causes problems in the analytical results, try the Ghost Trap DS, which removes impurities from organic solvents.
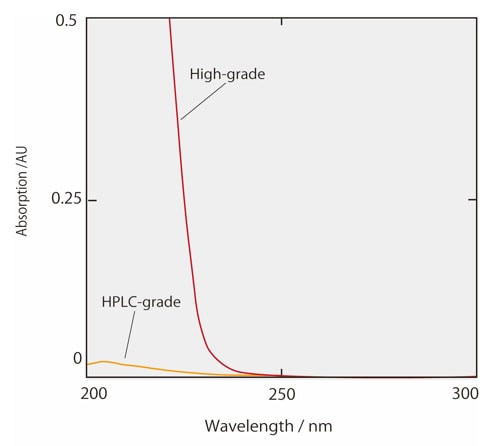
Fig.2 Absorbance spectrum of acetonitrile
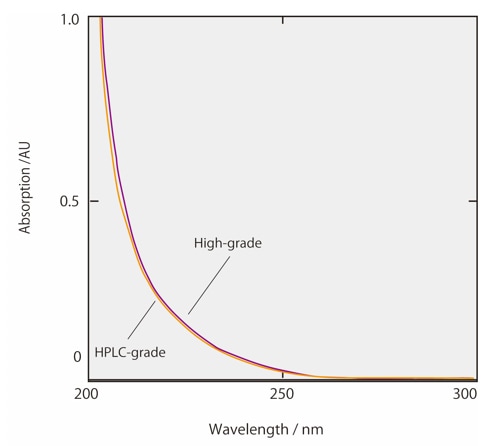
Fig.3 Absorbance spectrum of methanol
3.Elution Strength
Fig.4 shows an example separation of parabens, which is p-hydroxybenzoic acid, with ODS column. It can be seen that when acetonitrile and methanol are mixed with water in the same ratio, an acetonitrile mobile phase displays greater elution strength. The nomogram in Fig. 5 shows the ratios of methanol and acetonitrile to water with equivalent solvent strength, useful for approximate calculation of elution strength when changing between these solvents. If we have previously been using acetonitrile as the mobile phase with a ratio to water of 50/50 (v/v), when changing to methanol the equivalent ratio of methanol/water would be 60/40 (v/v).
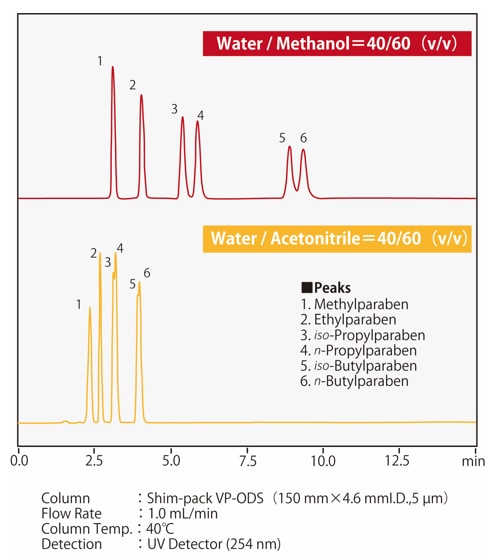
Fig.4 Comparison of the elution strength of methanol and acetonitrle
(p-hydroxybenzoic acid;parabens)
Fig.5 Nomogram of solvent strength for reverse-phase chromatography
(take from "Practical HPLC"Method Development”)
4.Separation Selectivity
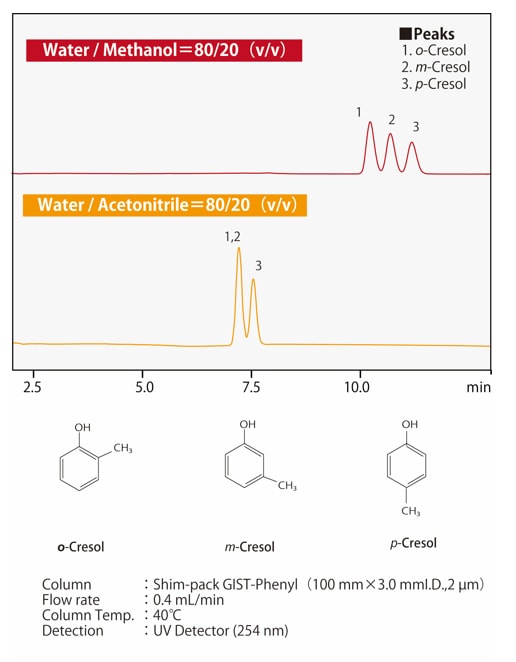 Fig.6 Difference in selectivity between methanol and acetonitrile
Fig.6 Difference in selectivity between methanol and acetonitrile(for positional isomers of cresol)
The separation selectivity of acetonitrile and methanol differ, but since selectivity depends on the properties of the dissolved compound, it is not the case that selectivity is always higher for one or the other. In the separation of positional isomers, phenyl columns may be the most appropriate amongst columns for reverse-phase chromatography. In addition to hydrophobic interactions, π-π interactions of the phenyl stationary phase contribute to separation. Fig. 6 shows an example of the separation of positional isomers of cresol. Acetonitrile (CH3-C≡N) has a triple C-N bond and therefore π electrons, whereas methanol (CH3-OH) has no π electrons. For a phenyl column, using methanol as the mobile phase allows π-π interactions, which improves the separation.
5.Retention Behavior
Methanol and acetonitrile have different chemical properties. Methanol and acetonitrile have different chemical properties. Methanol is a protic solvent, whereas acetonitrile is a non-protic solvent, so we know that their elution behavior will differ. If adequate separation cannot be obtained with an acetonitrile-based mobile phase, switching to a methanol-based mobile phase to change the elution order is one useful possibility for method development.
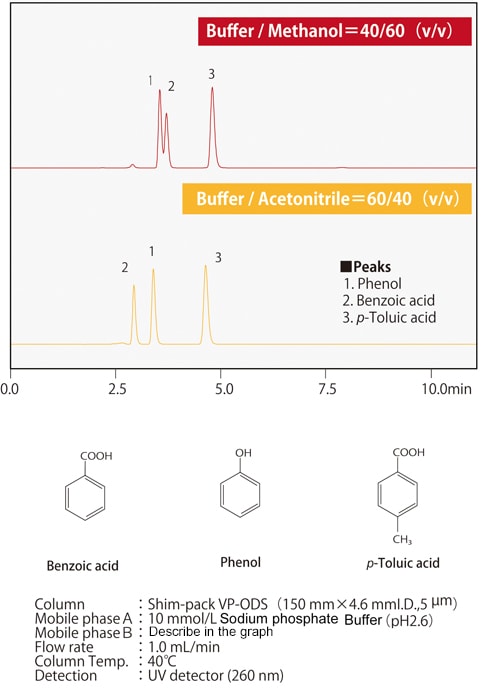 Fig.7 Differences in elution selectivity between methanol and acetonitrile
Fig.7 Differences in elution selectivity between methanol and acetonitrileFig. 7 shows an example of separation by methanol or acetonitrile of compounds in which one hydrogen atom of a benzene ring is substituted with a carboxyl group or a hydroxyl group. When the three compounds are equally retained, it can be seen that the elution order of phenol and benzoic acid changes depending on the solvent used. Depending on the column type, there may side-effects from polar functional groups from the packing material in addition to the chemically-modified functional groups such as ODS groups and C8 (octyl) groups. There are also cases where the organic solvents and side-effects from the functional groups together have a positive effect. Figs. 8 and 9 show the separation of 13 cephem antibiotics using a reverse-phase column and acetonitrile or methanol respectively, with the same analytical conditions in both cases. The retention and the elution order are different depending on whether acetonitrile or methanol is used. We also know that the elution order is different depending on the stationary phase. For example, when comparing ODS and C8 columns, the C8 column will generally have smaller retention values. However, due to the polar functional groups from the packing material, it is not simply the case that the retention is always smaller; the retention behavior also differs. Since the retention behavior is affected by these various factors, it is necessary to try different combinations of mobile and stationary phases in order to optimize analysis conditions.
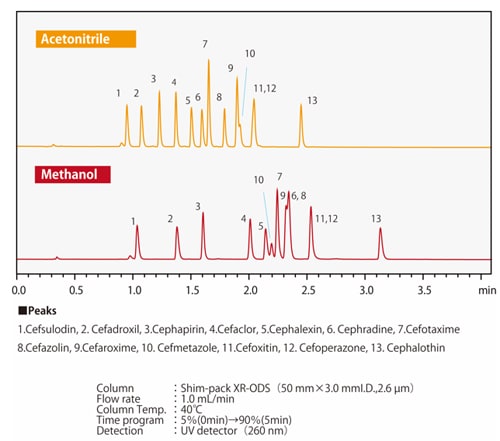
Fig.8 Separation of cephem antibotics using an ODS column
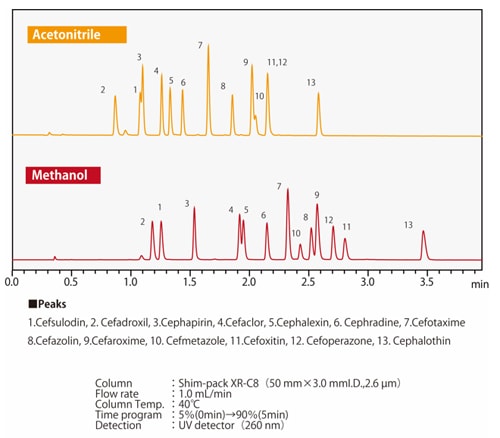
Fig.9 Separation of cephem antibotics using C8 column
6. Precipitation from mixing with a buffer
In reverse-phase chromatography, buffers are used with water-based mobile phases. They are mixed with organic solvents for use, but depending on the type of buffer and organic solvents, too high a quantity of organic solvents may cause the buffer salt to be precipitated. Tables 1 and 2 show whether precipitation occurs for mixtures of commonly-used buffers with acetonitrile or methanol respectively. The values in the table show the ratio (v/v) at which precipitation starts to occur. We can see that for some buffers there is no precipitation for either organic solvent, but in general methanol causes less precipitation.
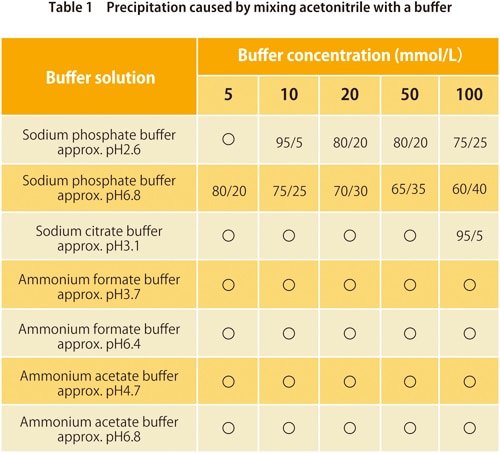
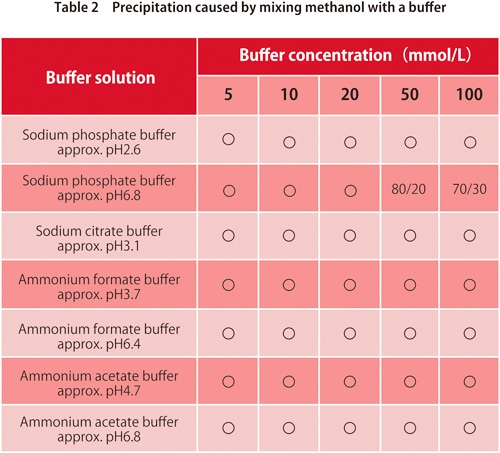
*Values shown in the table may vary depending on laboratory conditions.
** ”〇” means no precipitation.
7. Heat of reaction from mixing with water
For isocratic elution, water and organic solvent pre-mixed in the reserve bottle are used as the mobile phase. When mixed with water, methanol reacts exothermically. By contrast, acetonitrile reacts endothermically and therefore the temperature of the liquid will go below room temperature. As the acetonitrile mixture gradually returns to room temperature, bubbles tend to form in the liquid. Also, if the mixture is used as mobile phase before it has returned to room temperature, retention times will be faster and only stabilize as the liquid approaches room temperature. Meanwhile, methanol produces heat when mixed with water, which has a degassing effect. This means that preparing a water and methanol mixture as the mobile phase requires less care than in the case of acetonitrile.
Summary
Above we have introduced 7 key points in choosing between methanol and acetonitrile as the organic solvent in HPLC analysis. From the perspective of analytical workflow, there are differences in column pressure, UV absorption, and buffer compatibility, and when it comes to analytical separation, attention should be paid to elution power, separation selectivity and retention behavior. Understanding of these differences in chemical properties of methanol and acetonitrile, together with appropriate column combinations, reduces the risk of problems in HPLC analysis and improves the efficiency of method development.






