Q: What points need to be considered when replacing acetonitrile with methanol?
A:
Analytical Conditions:
- It is possible that separation from impurities and the elution order may change and so, in addition to the retention time of the target component, consider the separation patterns with respect to other components.
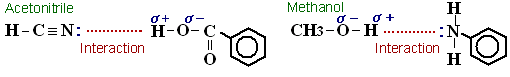
(Methanol is a protic solvent whereas acetonitrile is an aprotic solvent. Simultaneously with hydrophobic interaction, proton affinity influences analysis. For example, regarding compounds with functional groups that supply protons, such as –COOH groups, in a water/acetonitrile system, the compounds and the CN in acetonitrile (CH3CN) attract each other, which may speed up elution, but in a water/methanol system, this interaction is weaker and elution may consequently be slower. On the other hand, regarding compounds in which –NH2 groups are attached to aromatic rings, in a water/methanol system, the hydrogen in methanol and the lone electron pairs in the nitrogen in the -NH2 groups attract each other, and so elution may be faster than it is with a water/acetonitrile system.)
- If elution seems to be too fast overall, and separation seems to be poor, try decreasing the proportion of methanol. Conversely, if elution is too slow, try increasing the proportion of methanol.
- Refer to the instruction manual for your column to check whether or not methanol can be used with it.
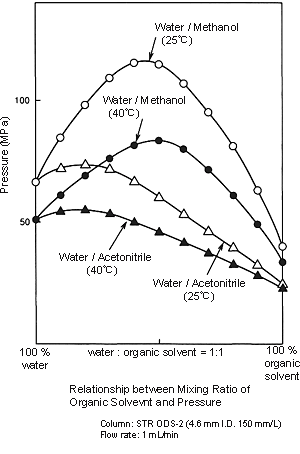
Pressure Applied to the Column:
- As shown in the figure on the right, the pressure applied when methanol is used is greater than that when acetonitrile is used. For example, if you switch from a 6:4 mixture of water and acetonitrile to a 4:6 mixture of water and methanol, the pressure increases by a factor of more than 1.5. Deliver mobile phase at about half the flow rate used in analysis, and then increase it within the maximum pressure range of the column while observing the effect. Care is required in gradient analysis because the pressure may increase as the proportion of organic solvent increases. To be safe, it is better to set “P.Max” for the pump. (This is the maximum pressure at which delivery is possible. Delivery stops if the pressure exceeds this setting.)
UV Detection:
- Because there is a large amount of absorption in the short wavelength region, depending on the detection wavelength, the baseline may be difficult to stabilize or there may be a large amount of noise. Consider this point in high-sensitivity analysis.
Solvent Replacement:
- Before delivering solvent with a high proportion of methanol, in order to prevent the precipitation of salt, first deliver a volume of methanol and water, mixed with the same ratio as the acetonitrile and water currently used, equivalent to about five times the column capacity (e.g., approx. 12.5 mL in the case of a 150 mm × 4.6 mm I.D. column), and then increase the proportion of methanol. If the proportion of organic solvent is the same, salt is normally not precipitated since methanol/water has greater salt solubility than acetonitrile/water.






