Oct 13, 2020
The Australian Imaging, Biomarkers and Lifestyle (AIBL), the National Center for Geriatrics and Gerontology (NCGG), and Koichi Tanaka Mass Spectrometry Research Laboratory at Shimadzu Corporation found a significant relationship between the plasma amyloid-β (Aβ) composite biomarker developed by the research team using immunoprecipitation-mass spectrometry (IP-MS) and cognitive decline. The results have important implications for the use of this biomarker in the detection of at-risk individuals.
The study was published in Journal of Alzheimer's Disease on September 29, 2020.
Study Overview
Alzheimer's disease is characterized by the pathological changes of abnormal accumulation of Aβ in the brain and the clinical symptoms of progressive cognitive decline. Studies on Aβ neuroimaging using positron emission tomography (PET) and cerebrospinal fluid (CSF) amyloid testing show that amyloid accumulation in the brain occurs several decades before the onset of Alzheimer's disease. This protracted preclinical Alzheimer’s disease period provides the study of Alzheimer's disease with a unique opportunity, but widescale assessment of Aβ in cognitively normal older adults is limited because Aβ biomarkers measured thorough PET or CSF are expensive and/or invasive.
Recently, we developed a plasma Aβ composite biomarker that combines plasma ratios of APP669-711/Aβ1-42 and Aβ1-40/Aβ1-42 measured by IP-MS. This biomarker can predict Aβ status with approximately 90% accuracy based on PET neuroimaging, and the area under the ROC curve (AUC) was 94 ~ 96% [ref. 1].
The aim of this study was to examine the relationship between this plasma Aβ composite biomarker and cognitive decline, a clinical manifestation of Alzheimer's disease.
In two independent cohort conducted by AIBL and NCGG, a total of 213 cognitively normal older adults underwent Aβ PET neuroimaging and provided blood samples. AIBL participants completed neuropsychological testing up to 5 timepoints, with a follow-up interval of 18-months and NCGG participants completed neuropsychological testing up to 4 timepoints, with a follow-up interval of 12-months. The episodic memory, execution function, language, and attention were assessed by the neuropsychological tests. Plasma was isolated from blood samples and plasma Aβ levels were measured by IP-MS with MALDI-TOFMS at Koichi Tanaka Mass Spectrometry Research Laboratory. We then examined the relationship between PET Aβ levels and plasma Aβ biomarker levels and changes in each cognitive function.
The results showed that plasma Aβ biomarker levels measured by IP-MS were significantly associated with faster cognitive decline in episodic memory and execution function, and that the magnitude of effects was comparable to that observed for the effects of PET Aβ levels on these same outcome measures.
These results suggest that this plasma Aβ biomarker by IP-MS will provide a useful prognostic marker for identifying individuals at-risk for Alzheimer's disease and for predicting the progression of clinical symptoms.
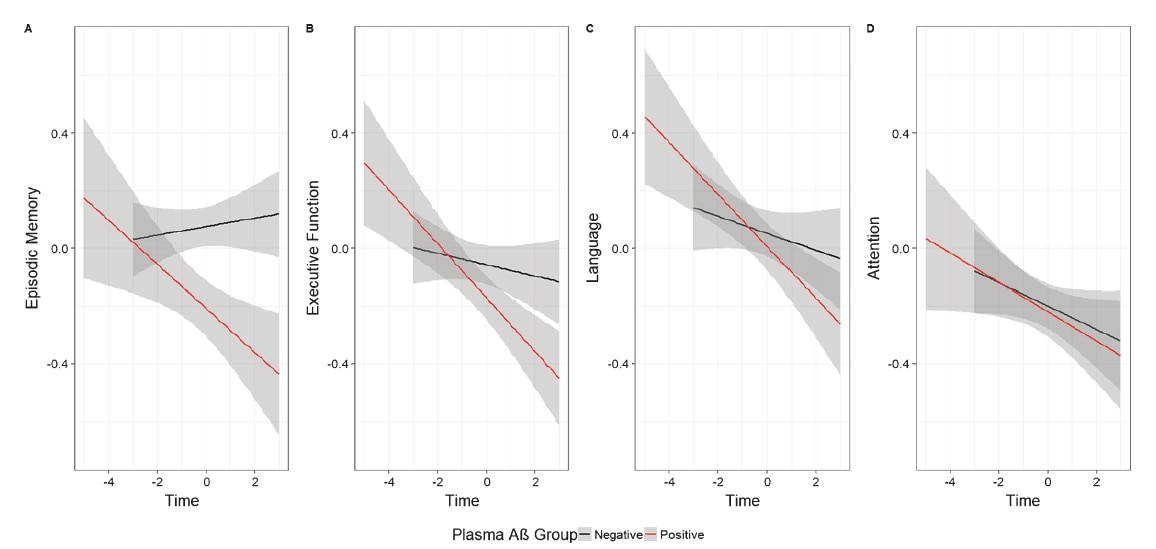
Fig. 1. Plasma Aβ+ is associated with faster decline in (A) episodic memory, and (B) executive function, but not (C) language or (D) attention
(shading indicates 95% confidence intervals). Reprinted from: Journal of Alzheimer's Disease, 77, 3, 1057-1065, 2020
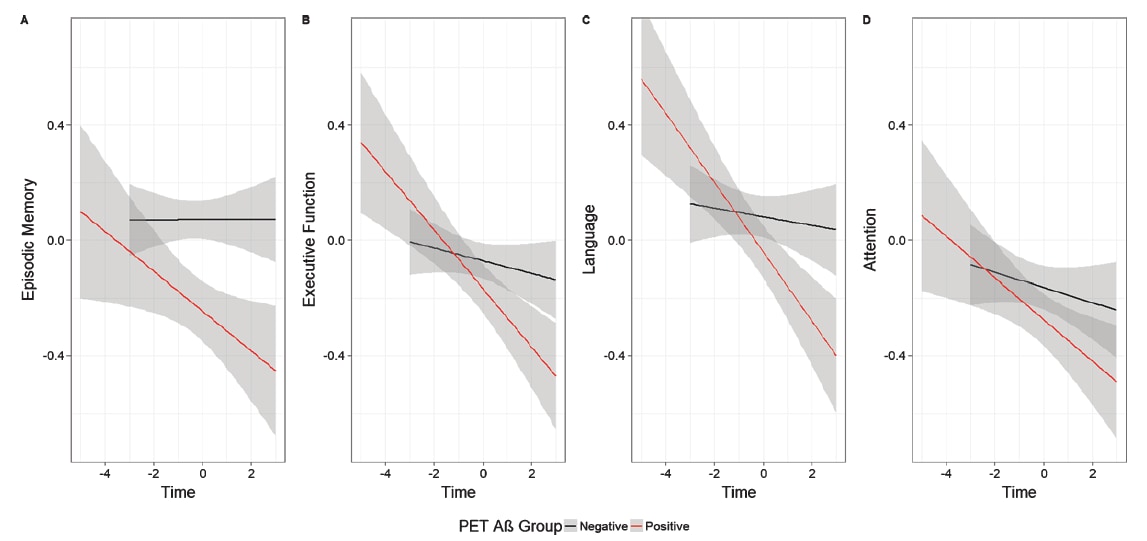
Fig. 2. PET Aβ+ is associated with faster decline in (A) episodic memory, (B) executive function, and (C) language, but not (D) attention
(shading indicates 95% confidence intervals). Reprinted from: Journal of Alzheimer's Disease, 77, 3, 1057-1065, 2020
【Paper Information】:
Yen Ying Lim, Paul Maruff, Naoki Kaneko, James Doecke, Christopher Fowler, Victor L. Villemagne, Takashi Kato, Christopher C. Rowe, Yutaka Arahata, Shinichi Iwamoto, Kengo Ito, Koichi Tanaka, Katsuhiko Yanagisawa, Colin L. Masters, Akinori Nakamura
"Plasma Amyloid-β Biomarker Associated with Cognitive Decline in Preclinical Alzheimer’s Disease"
Journal of Alzheimer's Disease, 77, 3, 1057-1065, 2020
DOI: 10.3233/JAD-200475
【References】:
1) Akinori Nakamura, Naoki Kaneko, Victor L. Villemagne, Takashi Kato, James Doecke, Vincent Doré, Chris Fowler, Qiao-Xin Li, Ralph Martins, Christopher Rowe, Taisuke Tomita, Katsumi Matsuzaki, Kenji Ishii, Kazunari Ishii, Yutaka Arahata, Shinichi Iwamoto, Kengo Ito, Koichi Tanaka, Colin L. Masters, Katsuhiko Yanagisawa
"High performance plasma amyloid-β biomarkers for Alzheimer's disease"
Nature, 554, 249-254, 2018
DOI: 10.1038/nature25456


