August 23, 2021 | News & Notices
Contributing to PCR Screening Tests for Mutant Strains
Release of SARS-CoV-2 Mutation Assay Core Kit and Primer/Probe Mixes
Shimadzu has released the SARS-CoV-2 Mutation Assay Core Kit, Primer/Probe Mix for N501Y, Primer/Probe Mix for L452R, and Primer/Probe Mix for E484K (all reagents for research purposes) in Japan. These products are based on the 2019 Novel Coronavirus Detection Kit (reagents for research purposes) released in April 2020. They can detect specific SARS-CoV-2 mutation sites directly from saliva or nasopharyngeal swab samples. Currently, these kits are only available in Japan, but preparations are underway to export them.
The SARS-CoV-2 Mutation Assay Core Kit (reagents for research purposes) consists of three reagents: a sample treatment solution, a reaction liquid, and an enzyme solution. Providing the primer/probe mixes for detecting the virus separately from this core kit was done to enable flexible support for the detection of a variety of mutations. The Primer/Probe Mix for N501Y, Primer/Probe Mix for L452R, and Primer/Probe Mix for E484K (all reagents for research purposes) are marketed for use in combination with the core kit to distinguish between the conventional strain and various mutant strains. The Shimadzu PCR Test Reagent Kit eliminates the troublesome and conventionally necessary processes of ribonucleic acid (RNA) extraction and purification. This reduces the number of people required for testing and shortens the entire testing process by half, from two hours or more to approximately one hour. In addition, testing is completed without manual procedures, which prevents human error.
It has been noted that the N501Y mutation site in the alpha strain (formerly the UK strain), the beta strain (formerly the South African strain), and the gamma strain (formerly the Brazilian strain) can increase the infectious capacity. It has been pointed out that the L452R mutation site in the delta strain (formerly the Indian strain) and the epsilon strain (formerly the California strain) can both increase infectious capacity and make it easier for the virus to break through immunity. In addition, the E484K mutation in the beta strain (formerly the South African strain), the gamma strain (formerly the Brazilian strain), and the theta strain (formerly the Philippine strain) can lower immunity and the effectiveness of vaccines in comparison with the conventional strain. Shimadzu is proactively developing primer/probe mixes to detect mutation sites, and will market these in succession, to respond quickly to the arrival of new mutant strains.
Caution: the SARS-CoV-2 Mutation Assay Core Kit, Primer/Probe Mix for N501Y, Primer/Probe Mix for L452R, and Primer/Probe Mix for E484K are intended for research use only. They are not recognized or certified as in vitro diagnostics or medical devices under Japan’s Pharmaceutical and Medical Device Act. They cannot be used for therapeutic diagnostic purposes or in related procedures. These kits require specialist equipment such as a real-time PCR device, dispensing pipettes, a thermal bath, and a small centrifuge, as well as the technology to handle samples and genes, so they are not sold to drug stores or other small retailers or individuals. Note that they are not compatible with the AutoAmp gene analyzer.
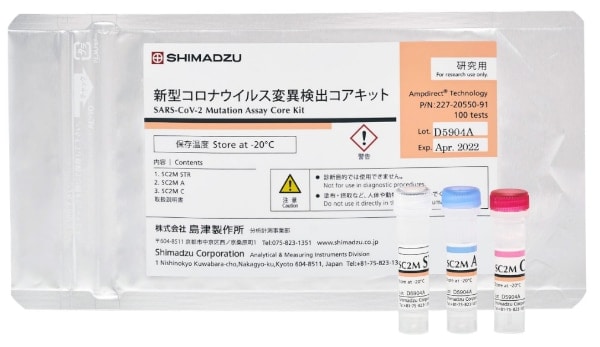
SARS-CoV-2 Mutation Assay Core Kit

Primer/Probe Mix for N501Y

Primer/Probe Mix for L452R
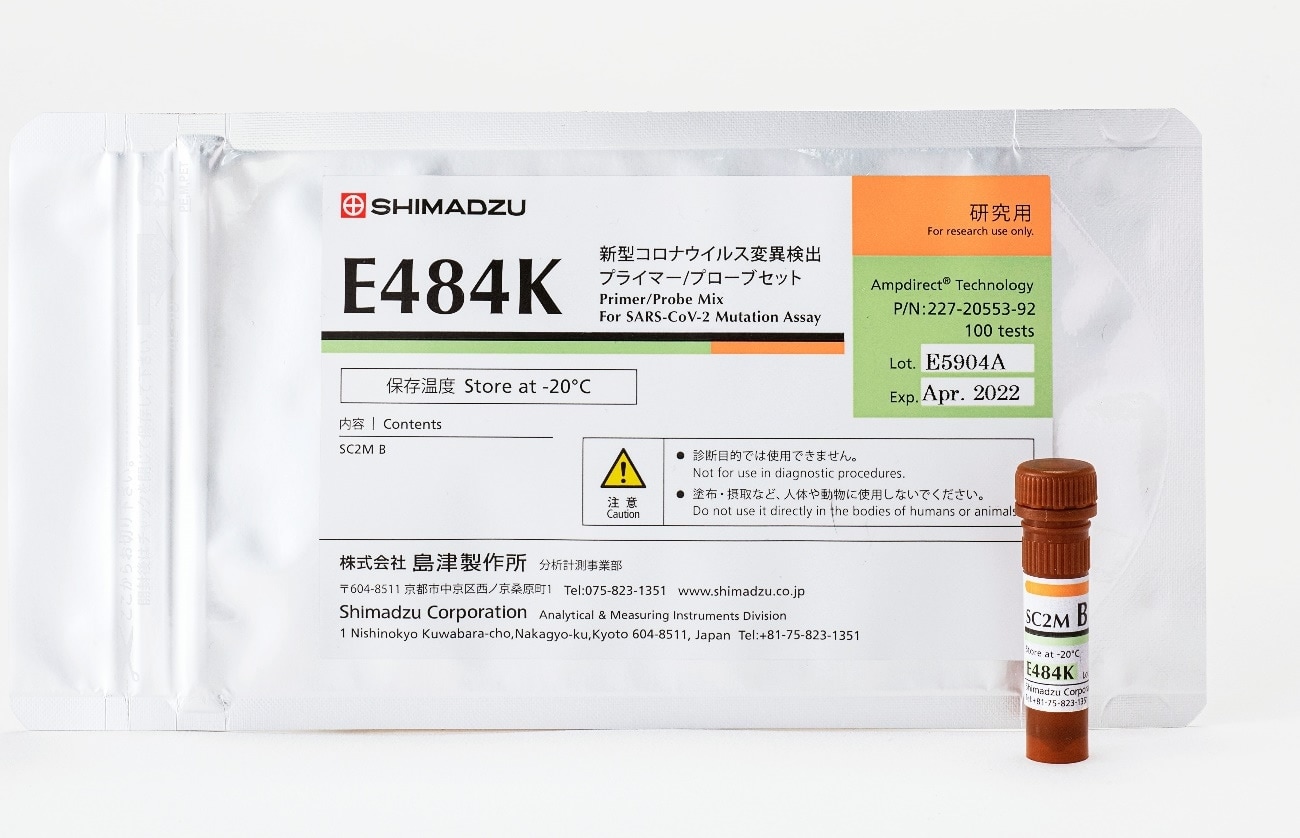
Primer/Probe Mix for E484K


