Intact Proteins
| CQAs observable at intact protein level | Analytical method |
|---|---|
| Aggregation, dimerization | Size-exclusion chromatography (SEC) |
| Deamidation, pyroglutamination | Charge-variant analysis (IEX) |
| Disulfide bond scrambling | Molecular weight measurement (LC-MS) |
| Major glycoforms | |
| Drug conjugation | Hydrophobic interaction chromatography (HIC) |

Accurate mass measurement helps to determine whether the correct protein sequence has been expressed with the expected post-translational modifications (PTMs). It also provides relative aboundance of different proteins or PTMs present in the same sample. A high resolution and high sensitivity mass spectrometer will facilitate this analysis. Shimadzu offers the best solution for a routine high-resolution accurate-mass analysis with the LCMS-9030, quadrupole time-of-flight mass spectrometer (Q-TOF LC-MS). It maintains its mass accuracy for days without requiring re-calibration or using internal standar
LCMS-9030 Q-TOF LC/MS System
Aggregate and Fragment Analysis
Protein aggregates are known to strongly induce immunogenic responses when administered, and aggregation is one of the most imortant quality attributes that must be monitored and managed at every step of process development and refinement. For example, in the initial stage of development, cell lines that have greater tendency to produce aggregates or degradants re screened out even if it showed a high secretion yield. Size-exclusion chromatography (SEC) is the method of choice for separating the major monomer fraction from size variants occurring by either aggregation or degradation. Shimadzu supplies the range of SEC columns that are useful particularly in increasing the throughput needed for the screening analysis.
Size-exclusion chromatography of mAb
Column : shim-Pack Bio Diol-300
Flow rate : 0.2 mL/min
Sample : Humanized monoclonal lgG1
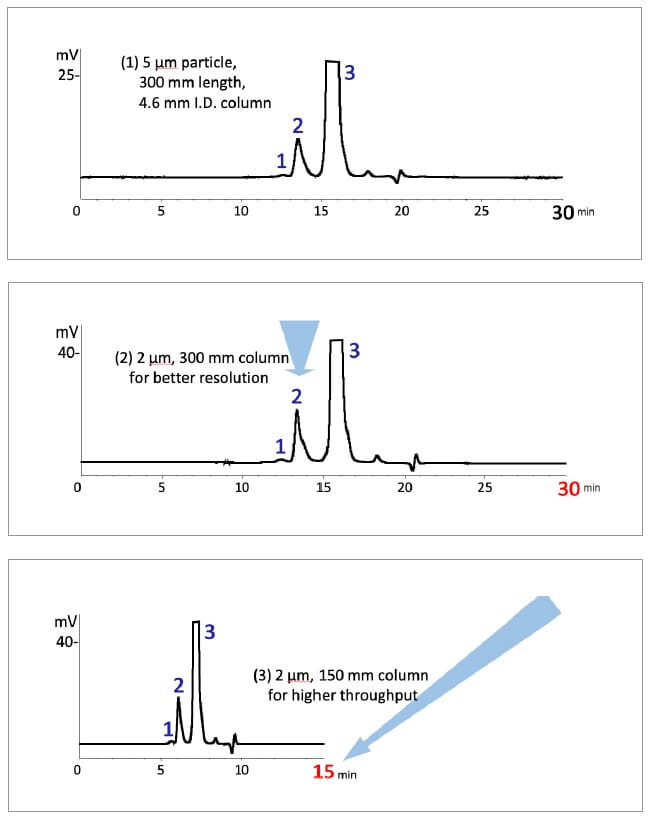
Here, the chromatograms compare the SEC separation profile of mAb acquired by Shim-pack Bio Diol-300 columns of different dimension at a constant frow rate. Reduction of particle size (5μm to 2μm) resulted in increased peak resolution, which in turn gave the room to reduce the analysis time by using a shorter column (30 minutes to 15 minutes).
| Column | N (3) | Rs (1,2) | Rs (2,3) |
|---|---|---|---|
| (1) 5μm, 300×4.6mm | 8,500 | 0.88 | 2.67 |
| (2) 2μm, 300×4.6mm | 16,200 | 1.17 | 4.15 |
| (3) 2μm, 150×4.6mm | 8,700 | 0.85 | 2.75 |

Shim-pack Bio Series
Charge Variant Analysis
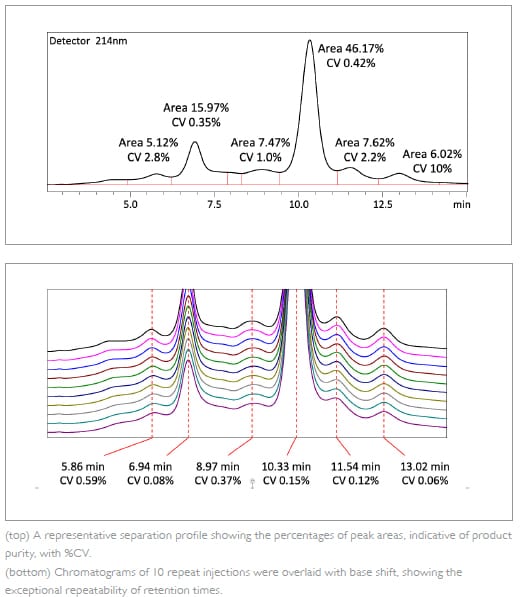
Modification, isomerization or cleavage occurring on a charged amino acid residue alters the overall charge of the protein, affecting its structure, binding affinity and stability. Hence charge-related heterogeneity is an important quality attribute of mAb. The profile of charge variants can be evaluated by ion exchange chromatography (IEX), and this is repeatedly monitored throughout the developmental workflow.Repeatability of elution profile strongly depends on the robustness of the HPLC system to sustain precision and accuracy in the presence of strong salts needed for IEX.
Here, mAb sample was separated by cation-exchange chromatography and repeatability of 10 consecutive injections was evaluated. The results shown demonstrate that the system precisely sustains the baseline and elution profile in the presence of 0.2 M NaCl.
Cation-exchange chromatography of mAb
HPLC System : Prominence-i
Mobile phase : 0.02 M sodium phosphate and 0.2 M NaCl
Flow rate : 1.0 mL/min
Sample : mAb (Trastuzumab)


