Analysis of Glycopeptides of Monoclonal Antibody Using MALDI-7090 High-Resolution MALDI-TOF MS
Antibodies that are utilized in biopharmaceuticals are often subjected to glycan modification. This glycan is a molecule with high structural heterogeneity consisting of intricately coupled monosaccharides, such as glucose and mannose, and that complex structure is known to play an important role in the functional regulation of proteins. However, information such as the complex structure of the glycan and the site at which the glycan is attached to the protein is not written into the gene beforehand. Rather, it is information that is formed based on the actions of many glycosyltransferases during the process of protein biosynthesis. Therefore, depending on the state of growth of the cells that produce antibodies, it is not uncommon to see such phenomena as the formation of glycans having different structures in spite of the same protein backbone, or the formation of the glycan bond at an unexpected site. Therefore, when an antibody is biosynthesized as a biopharmaceutical, elucidation of both its structure and binding site is important. Mass spectrometers are now regularly used for ascertaining both the binding site as well as the structure of glycans.
Here, using the MALDI-7090 high-resolution MALDI- TOF MS with its unique 20 keV high energy fragmentation capability, we introduce an example of analysis to determine the structure and binding site of a glycan bound to an antibody.
Preparation of Glycopeptides from a Glycoprotein
First, using a commercially available monoclonal antibody as the sample, reduction and alkylation was conducted in the solution. Then, trypsin was added to this solution, allowing enzymatic digestion to continue for 2 hours. The enzyme-digested solution was then added to a spin column packed with Sepharose CL4B gel previously equilibrated using butanol: ethanol:water (4:1:1), and the solution was allowed to interact with the gel, permitting adsorption of the glycopeptide to the gel. Then, by washing with the equilibrated solution to remove non-glycosylated peptides, glycopeptides were recovered using aqueous ethanol solution. The recovered glycopeptides were spotted on a target plate using 2,5-dihydroxybenzoic acid (DHB) as the matrix, and analyzed using the MALDI-7090.
MS Analysis of the Glycopeptide Fraction
The mass spectrum of the recovered glycopeptide fraction is shown in Fig. 1. Of the detected signals, m/z 2405.72, 2430.75, 2567.72, 2592.73, 2633.74, 2795.74 and 2957.74 were considered to be signals derived from glycopeptides. Because glycans have a non-uniform structure, multiple different glycan structures are present in one peptide backbone. Since a signal in MADLI-TOF MS is associated with a monovalent ion, it is possible to look at mass differences between peaks, and if they match the mass of a glycan moiety, then those peaks are likely glycopeptides.
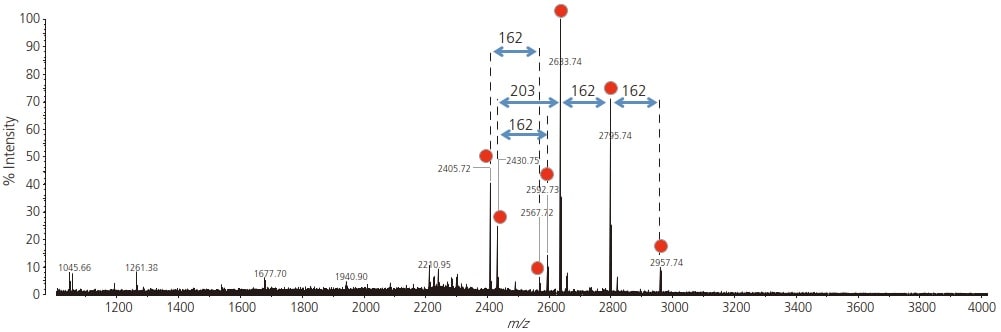
Fig. 1 Mass Spectrum of the Glycopeptide Fraction from Monoclonal IgG Glycopeptide signals are shown by red circles.


