Post-Translational Modification Analysis | Phosphorylation Analysis by MALDI-TOF MS (3)
Post-Translational Modification Analysis
Phosphorylation Analysis by MALDI-TOF MS (3)
■ Phosphopeptide Enrichment Using TiO2
Phosphorylation of proteins is one type of post-translational modification. It is important in the control of biological functions. Recently, mass spectrometry has been applied to the analysis of phosphorylation sites. However, due to the low ratio of phosphorylation and the significant decrease in ionization efficiency due to phosphorylation, it is often difficult to analyze mixtures in their original state.
Phosphorylation analysis using a combination of TiO2-based phosphopeptide enrichment and MALDI-MS/MS (seamless PSD) is introduced below.
Fig. 1 shows the effect of enrichment by TiO2. The phosphopeptides were specifically enriched, and the phosphopeptide (m/z 1660) that could not be detected using desalting alone was observed.
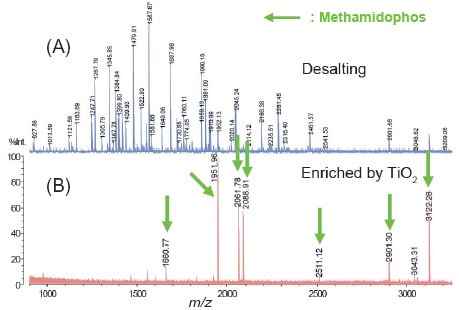
Fig. 1 Phosphopeptide Enrichment Using TiO2: (A) Desalting by C18 Tip, (B) Enrichment by TiO2
AXIMA Performance MALDI-TOF MS

The AXIMA Performance is one of the most powerful tools in mass spectrometry, delivering information-rich spectra with greater sensitivity and higher confidence in identification. It is an extremely versatile and powerful TOF-TOF system, integrating workflows for a diverse range of analytical needs.
- From high energy MS/MS of proteomics and other biological and organic samples to uncompromised analysis of high mass intact proteins
- True high energy MS/MS - CID with a laboratory frame collision energy of 20keV
- Optimal precursor ion selection resolution using revolutionary gating technology
- Outstanding sensitivity - uncompromised design, to ensure no MS/MS signal is discarded


