Post-Translational Modification Analysis | Analysis of Synthetic Protein Prenylation by Mass Spectrometry
Post-Translational Modification Analysis
Analysis of Synthetic Protein Prenylation by Mass Spectrometry
The importance of protein post-translational modification analysis has heightened in recent years.
Protein prenylation is a C-terminal lipid modification, many of which have been recognized in low-molecular-weight GTP-binding proteins such as Ras. It is known to play an important role in protein-membrane and protein-protein interactions
An effective analysis method for protein prenylation using a combination of the Transdirect insect cell cell-free protein synthesis kit and MALDI-TOF mass spectrometer is introduced below.
Protein prenylation : The 15-carbon farnesyl group or 20-carbon geranylgeranyl group isoprenoid is transferred to the cysteine residue near the C-terminal by prenyltransferase. (See diagram below.)
Farnesyltransferase (FTase) or geranylgeranyltransferase I (GGTaseI) recognizes the C-terminal-Cys-Ali-Ali-COOH motif (where Ali is an aliphatic amino acid) and transfers the farnesyl group or geranylgeranyl group to the cysteine residue with prenyl pyrophosphate as the donor.
We analyzed human-derived RhoC (involved in signal transmission) in which geranylgeranylation is known to occur as the model protein.
■ Test Flow
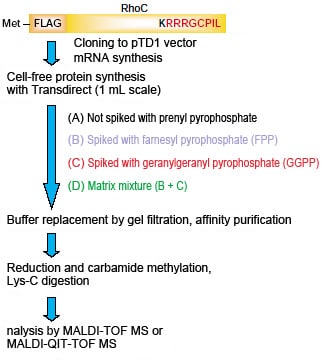
■ Results
Not spiked with prenyl pyrophosphate:
Only carbamide-methylated C-terminal peptide fragments detected.
Spiked with FPP:
Partially farnesylatedm/z1174.75 peptide detected.
Spiked with GGPP (including matrix mixture):
Geranylgeranylatedm/z1242.83 peptide detected.
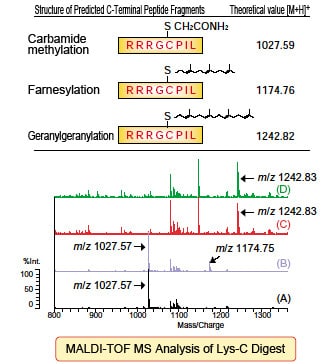
The results above show that Transdirect retains prenyltransferase that offers the activity required for protein prenylation. In combination with a mass spectrometer, it can be seen that this method provides a powerful tool for prenylation analysis.
AXIMA Resonance is a MALDI-QIT-TOF mass spectrometer that provides powerful support for proteomics and sugar chain analysis.
It incorporates a quadrupole ion trap (QIT) for fmol-order MSn measurements of the ions generated by MALDI.
These features provide structural information in addition to molecular mass, making MALDI-QIT-TOF MS widely used for proteomics, biochemistry, pharmaceuticals, medicine, synthetic chemistry, and structural analysis of organic compounds.


