Analysis of Irbesartan Using an Integrated LC System - Nexera-i MT
Irbesartan and amlodipine besilate tablets are newly listed in Supplement 1 to the 17th Edition of the Japanese Pharmacopoeia (JP), which specifies a column packing particle size of 2.2 μm meaning that a UHPLC needs to be used. Due to
the pressure tolerance of the instrument, applying the analytical conditions may not be possible as described. Under such circumstances, Notification No. 0331-1, issued by the Director of the Evaluation and Licensing Division, Pharmaceutical Safety and Environmental Health Bureau, MHLW, states that, on the understanding that the monograph of the drug being tested shall be revised, an application for
approval of such drugs is possible by employing analytical conditions using conventional liquid chromatography on the basis of appropriate analytical validation data. The notification is considered to be indicating that it is acceptable to change the
JP listed conditions to conventional (HPLC) conditions.
This article introduces a system suitability test and analysis using the listed conditions transferred to an HPLC conditions for quantitative determination of irbesartan in irbesartan and amlodipine besilate tablets that are newly listed in Supplement 1 to the 17th Edition of the JP, using a Shimadzu integrated LC system, Nexera-i MT.
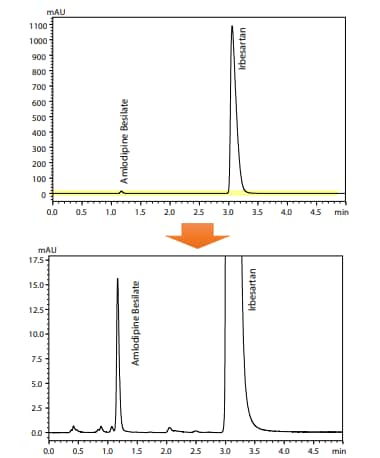
Fig. 1 Results of Irbesartan (and Amlodipine Besilate) Analysis
i-Series
Amid increasing calls for improved work efficiency and a more flexible working style, ideas of LC analysis are changing. The time has come for an HPLC that delivers rugged, reliable results with less frequent interaction by the analyst. The new, integrated i-Series LC system maintains the excellent performance of its predecessor while addressing the need for automation efficiency.


