Analysis of Residual Solvents in Drugs (Headspace GC)
To reduce the burden of analyzing residual solvents in drug products for drug approval applications by manufacturers, ICH decisions are leading to a standardization of the Japanese Pharmacopoeia (JP), U.S. Pharmacopoeia (USP), and European Pharmacopoeia (EP) analytical methods. Formerly, the USP stipulated the methods for analyzing residual solvents in drugs in USP <467> Organic Volatile Impurities. However, it was completely revised based on the analysis method in the EP, and the USP chapter title was accordingly changed to Residual Solvents. The FDA has also issued the following recommendations in the draft guidance "Residual Solvents in Drug Products Marketed in the United States."
- To sell products listed for the official analytical method in the United States, they must meet the requirements of USP <467> Residual Solvents, whether or not they are approved under a New Drug Application (NDA) or Abbreviated New Drug Application (ANDA).
- Instead of testing the final drugs, testing can be performed on the drug ingredients and drug additives.
- Apart from analysis methods listed in USP <467>, it is acceptable to use another analytical procedure properly described and validated according to the CGMP (standards for manufacturing control and quality control of drugs) requirements.
Analysis by the GC-2010 Plus with a Headspace Sampler
The USP, EP, and "Guidelines for Residual Solvents in Drug Products" notified by the former Japanese Ministry of Health and Welfare classify solvents according to toxicity as Class 1 (solvents to be avoided), Class 2 (solvents whose residual amounts should be limited), and Class 3 (solvents with low toxic potential).
The analysis of Class 1 solvents (aqueous solution) and Class 2 solvents (aqueous solution) is introduced below.
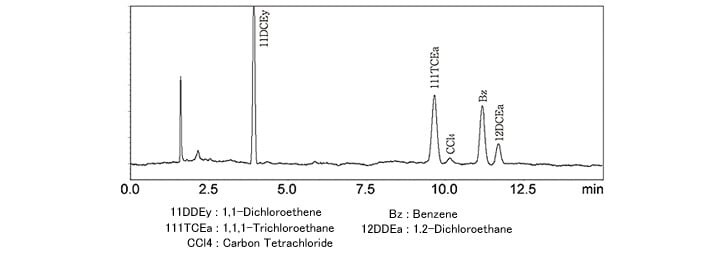
Fig. 1 Analysis of Class 1 Solvent Standard Sample (Aqueous Solution, Procedure A)
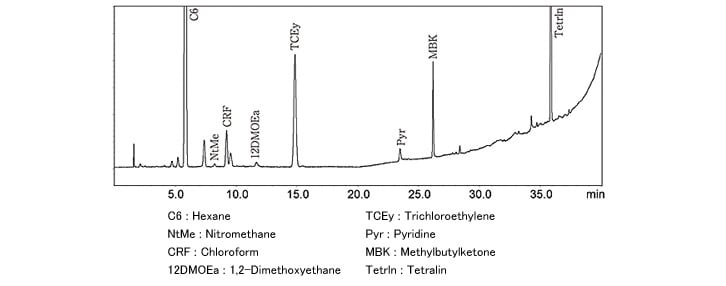
Fig. 2 Analysis of Class 2 Solvent Standard Sample (Aqueous Solution, Procedure A)
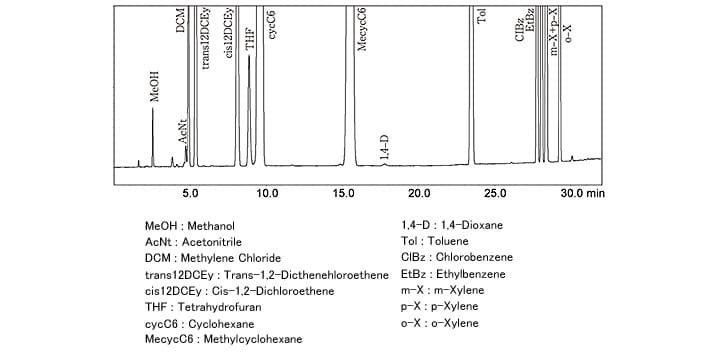
Fig. 3 Analysis of Class 2 Solvent Standard Sample (Aqueous Solution, Procedure B)
Nexis GC-2030
Nexis GC-2030, Shimadzu's premier gas chromatograph, offers a modern approach to a classic chromatographic technique. Designed with the user in mind, new innovative features, exceptional performance and high-throughput capabilities will elevate your lab to the next level.


