Improving the Data Integrity of Spectrometers
Data Integrity and Report Set
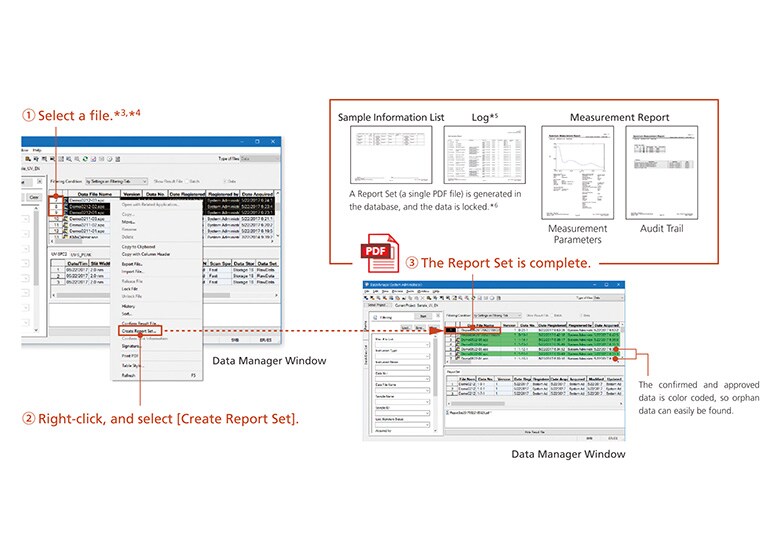
Data integrity refers to the completeness of data, with nothing missing or inconsistent. In other words, not only the data itself but also the metadata (the results of work that require human intervention, such as specifying conditions and analyzing analysis data) must be presented in a visible form, and verified together with the data. This is achieved by the Report Set.
Why Data Integrity Is Important?
The following warning letters have been issued by the FDA, demanding swift response regarding analytical instruments that do not support data integrity.
| Warning Letter: 320-18-55 Issue Date: May 23, 2018 You did not have procedures for reviewing audit trails or electronic data for the Fourier-transform infrared spectroscopy or ultraviolet systems. |
| Warning Letter: 320-17-25 Issue Date: February 24, 2017 Our investigator observed that your laboratory systems lacked controls to prevent your staff from altering or deleting electronic data. Analysts manipulated and deleted audit trails. You lacked adequate controls for all HPLC, gas chromatography, and ultra-violet systems. |
| Warning Letter: 320-17-01 lssue Date: October 13, 2016 In response, to this letter, provide details of your retrospective review of HPLC and other laboratory data, such as Fourier transform infrared spectroscopy, gas chromatography, UV spectrophotometry, and (b)(4) analyzer data. |
| Warning Letter: 320-15-09 lssue Date: April 6, 2015 You lacked controls to prevent the unauthorized manipulation of your laboratory’s electronic raw data. Specifically, your infrared (IR) spectrometer did not have access controls to prevent deletion or alteration of raw data. |
Features
-
This function gathers operational information (operations and settings that require human intervention) distributed within the software, and collects it in a single report.
News / Events
-
Particle Analysis System for Microplastics has been released
This system can quickly calculate the number of particles, area, volume, mass, and individual particle qualities of microplastics based on the measurement results from an infrared microscope or an infrared Raman microscope.
-
UV-Vis Spectrophotometer UV-2600i Plus/UV-2700i Plus has been released
Accommodating a wide range of accessories, UV-2600i Plus/UV-2700i Plus excels in diverse applications, measures slight absorbance differences, and ensures robust data management and compliance.
-
UV-Vis Spectrophotometer UV-1900i Plus has been released
Experience unmatched precision and ease with UV-1900i Plus. Featuring a refined user-friendly interface, ultra high-speed scanning, and comprehensive support functions, UV-1900i Plus ensures accurate and efficient measurements for all your needs.
-
TEC MCT (Peltier Cooled MCT) Detector is now available
Equipping the AIMsight Infrared Microscope or the AIRsight Infrared/Raman Microscope with the TEC MCT (peltier cooled MCT) detector makes it possible to obtain infrared spectra without using liquid nitrogen.
-
FTIR TALK LETTER Vol. 43 has been published
The article about the Introduction to the Spectrum Advisor can be used on all Shimadzu FTIR systems controlled by LabSolutions IR. Learn the key points of infrared spectral analysis for aliphatic unsaturated hydrocarbons and aromatics.
-
Analytical Solutions for Microplastics
Shimadzu provides analytical and measuring instruments for the study of a variety of plastic materials: for R&D, characteristic evaluation of raw materials, quality control for plastic products, and deterioration analysis. With these diverse techniques, Shimadzu provides optimal solutions for microplastics research.