Pharmaceutical Elemental Impurities Analysis System - Options
Energy Dispersive X-ray Fluorescence Spectrometer
FDA 21 CFR Part 11-Compliant System
The EDX-7000 is also compliant with FDA 21 CFR Part 11. With this option, user administration, system administration, and data management can be performed with LabSolutions.
PCEDX for Part 11 DB (Standalone Database Edition)
Contents
- Media (PCEDX for Part 11 installer, LabSolutions DB connection kit, instruction manuals, PDF creation tool)
- Software license installation manual
PCEDX for Part 11 CS (Client Server Edition)
Contents
- Media (PCEDX for Part 11 installer, LabSolutions CS connection kit, instruction manuals)
FDA 21 CFR Part 11 Compliance Features Needed in Pharmaceutical Industry
The following functions compliant with FDA 21CFR Part 11 are provided.
- Security Function
- User Right’s Management
- Validation Function
- Operation and Audit Trail Log Output Functions
- PDF Data Output
LabSolutions DB/CS Data Management - Higher Efficiency
Contents
All analytical data including data obtained from other analytical instruments is managed on a database on the server computer, allowing data to be loaded and managed on any computers on the network.
Pharmaceuticals Impurities Analysis Method Package
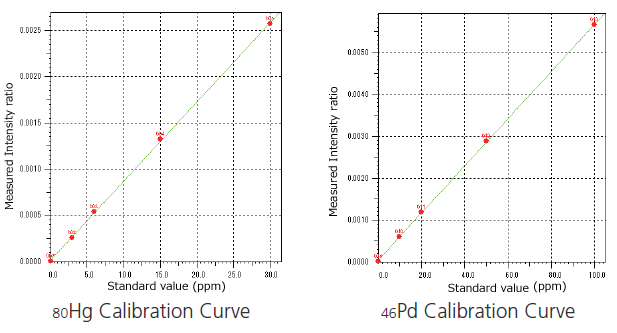
This package provides analysis methods to support the four elements in Class 1 as stipulated by ICH Q3D, the three elements in Class 2A, and five elements from among the Class 2B elements. Analysis is performed by creating a calibration curve with a specified standard mixture solution and solutions made by diluting it.
Pharmaceuticals Impurities Analysis Method Package
Contents
- Conditions media (pharmaceuticals impurities analysis conditions file) (overlap correction measurement conditions file)
- Instruction manual for pharmaceuticals impurities analysis (PDF format)
Analysis for Cd, Pb, As, Hg, Co, V, Ni, Pd, Ir, Rh, Ru, and Pt
This accommodates 12 important elements requiring risk assessment from among the 24 elements stipulated by the ICH Q3D.
Calibration curve conditions using the background internal standard correction method
This method corrects quantitative determination errors arising from differences in sample quantities, forms, and matrices.
Note: The standard mixture solution used for creating the calibration curve is not included in the package. Purchase the following separately.
SPEX CertiPrep (USA)
- XSTC-2046 (Cd, Pb, As, Hg, Co, V, Ni)
- USP-TXM 4 (Pd, Ir, Rh, Ru, Pt)


