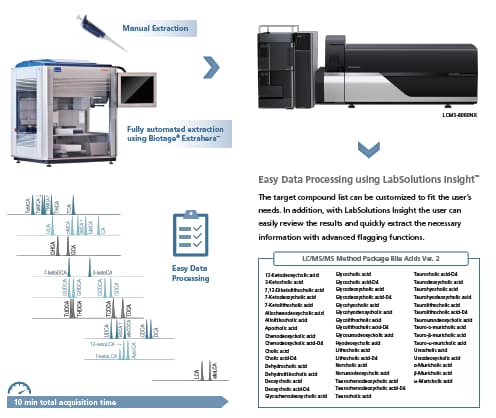
LC/MS/MS Method Package for Bile Acids
For LabSolutions™ LCMS
LC/MS/MS Method Package Bile Acids Ver. 2
Ready-to-use method for analysis of 49 Bile Acids
This Method Package allows comprehensive analysis of 49 Bile Acids from plasma, urine and feces. Bile Acids are essential for cholesterol metabolism and play a key role in absorption of fats in the small intestine. Additionally, they serve as signaling molecules that regulate metabolism. Their concentrations are used as markers of several diseases. Acute and chronic liver failure concern more than 12 million people worldwide, estimated by the World Health Organization, accounting for approximately 2 million deaths per year. Blood enzyme activity test (ALT, AST, etc.) and total bile acids assay (TBA) are widely conducted as examinations for the early diagnosis of liver diseases and liver function evaluation. More recently, monitoring of individual bile acids (IBA) was proposed for deeper pathologies characterization. However, bile acids wide structural diversity, associated with chemical similarities, leads to great challenges to analyze them.
This Method Package was developed to provide users a complete solution to perform routine analysis of selected primary, secondary and conjugates bile acids, in various matrices such as plasma, urine and feces.
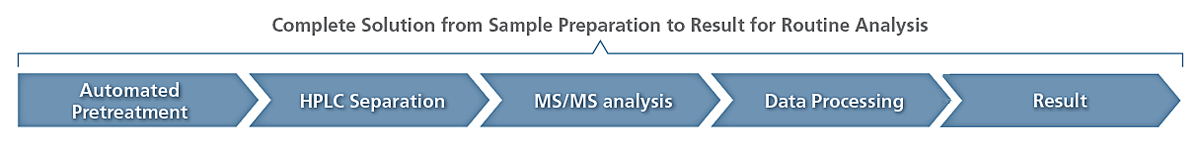
- Example pretreatment protocols (including automation) for plasma, urine and feces samples,
- Robust chromatographic conditions for separation of isobaric species within a 10-min acquisition time,
- Optimized MS/MS parameters for all targeted bile acids and their conjugates (up to 3 MRM transitions),
- Optimized MS/MS parameters for 10 commercially available internal standards, covering all classes,
- Ready to use method files for LCMS-8050/8060 and LCMS-8060NX,
- Instruction manual for method installation.
Features
News / Events
-
Shimadzu has released the LCMS-8065XE
The new LCMS-8065XE is a triple-quadrupole mass spectrometer with EVOLVED, EFFICIENT, and EXACT capabilities. These exceptional capabilities ensure high reliability and enhanced productivity, empowering the laboratory for the future.
-
Latest issue of Shimadzu Journal, featuring Environmental Analysis, has come out.
This issue showcases advanced technologies and research tackling the global challenges posed by PFAS. As part of Shimadzu’s ongoing commitment to sustainability and problem solving, we strive to reduce environmental impacts and build a better future.
-
New High Resolution Accurate Mass Library for Forensic Toxicology
Perform forensic toxicology screening for drugs of abuse, psychotropic drugs, pharmaceuticals, pesticides, and natural toxins using this high-resolution accurate mass database.
-
Shimadzu has released the Shim-vial™ H glass, S glass.
Shimadzu provides high-quality vials that thoroughly eliminate these risks by visually inspecting each vial, allowing them to be used with confidence.
-
INTERNATIONAL MASS SPECTROMETRY CONFERENCE 2024
Visit the Shimadzu booth at the International Mass Spectrometry Conference (IMSC) 2024.
-
Metabolomics 2024
Shimadzu Lunch Presentation at Metabolomics 2024
Date: June 20th, 2024 (Thursday)
Time: 12:25 – 1:25 p.m.