

TOC Academy

Oxidation Methods for TOC Analyzers
Two methods are used to oxidize organic matter, either combustion oxidation or wet oxidation.
Combustion Oxidation Method
One of the main features of this method is its ability to efficiently oxidize organic carbon matter that is otherwise resistant to decomposition, such as carbon matter that contains insoluble or macromolecular organic substances.
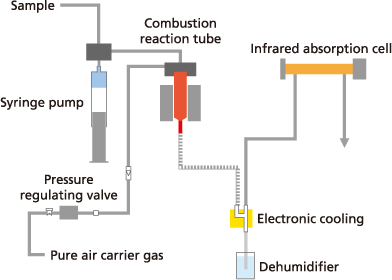
The sample is injected into a high-temperature (650 to 1,200 °C) combustion furnace to incinerate all organic carbon in the sample and measure it as fully oxidized carbon dioxide.
Because samples are combusted at a high temperature, the method enables complete oxidative dissociation of carbon even in persistent organic matter, or carbon in suspended substances or other water-insoluble particulate organic matter.
Due to the simplicity of using heat/combustion as the principle for oxidation, the method requires no reagents for pretreatment or posttreatment processes.
Wet Oxidation Method
With this method, an oxidizing agent is added to samples to chemically decompose carbon in organic matter for measurement as carbon dioxide. Though heat (up to 100 °C) or ultraviolet irradiation can be applied to promote the oxidation reaction, the ability of the chemical reaction to oxidatively decompose matter is weaker than combustive oxidation, which tends to result in lower carbon recovery rates from suspended or other particulate organic matter, or persistent substances.
Therefore, due to its superior oxidative reaction, the combustion oxidation method is commonly used to measure TOC levels in environmental water, factory effluent, and other such samples, where water samples often contain large amounts of insoluble organic carbon.


