The HIC-ESP is a new anion suppressor ion chromatograph with built-in electrodialytic suppressor, boasting the same low carryover and excellent injection precision characteristic of Shimadzu HPLCs to bring you highly-reliable results.
Ion Exchange Chromatography
What Is Ion Exchange Chromatography?
- Ion exchange chromatography (IEX) is a separation method that exploits the difference in charge states between the mobile phase and analytes within a chromatography column. This technique enables the analysis and purification of ionic compounds by using stationary phases modified with charged functional groups that attract oppositely charged molecules through electrostatic interactions.
Ion exchange chromatography (IEX) is a separation method using the difference of charge states between the mobile phase and analytes from the stationary phase in the column. It is mainly used for the analysis of ionic compounds.
There are two types of IEX; anion exchange chromatography and cation exchange chromatography, and the type used depends on the ionic strength of the ion exchange group. Table 1 shows typical functional groups per IEX stationary phase type. Strong ion-exchange functional groups can always be ionized, while ionization conditions of weak ion-exchange groups can vary depending on the pH of the mobile phase. The appropriate stationary phase is selected according to the charge and properties of the analyte.
Note that when ion exchange chromatography is applied, cations and anions cannot be separated within a single analysis. -
Table 1. Typical Ion Exchange Functional Groups Used In the Stationary Phase
Ionic Strength Typical Functional Groups Cation Exchange Chromatography Strong (SCX) Sulfonic acid Weak (WCX) Carboxylic acid Anion Exchange Chromatography Strong (SAX) Quaternary ammonium Weak (WAX) Tertiary amine
How Ion Exchange Chromatography Works
The Separation Mechanism
Fig.1 shows the workings of anion exchange chromatography.
In the stationary phase of anion exchange chromatography, packing materials modified with positively charged ion-exchange groups are used. When the mobile phase (eluent) enters the column, it absorbs onto the ion exchange groups of the stationary phase due to electrostatic attraction. The mobile phase continuously introduces anions into the column, resulting in a repeated adsorption and desorption of anions between the stationary and mobile phases, i.e. a state of equilibrium.When a sample containing anions is introduced into the column during this state, the anions in the sample are adsorbed through electrostatic interactions with the functional groups of the stationary phase. At the same time, the anions from the mobile phase that were previously adsorbed on the stationary phase are desorbed. The sample anions adsorbed on the stationary phase are then desorbed and adsorbed onto the next ion exchange group. This phenomenon is repeated until the sample anions completely move through the column and are eluted.
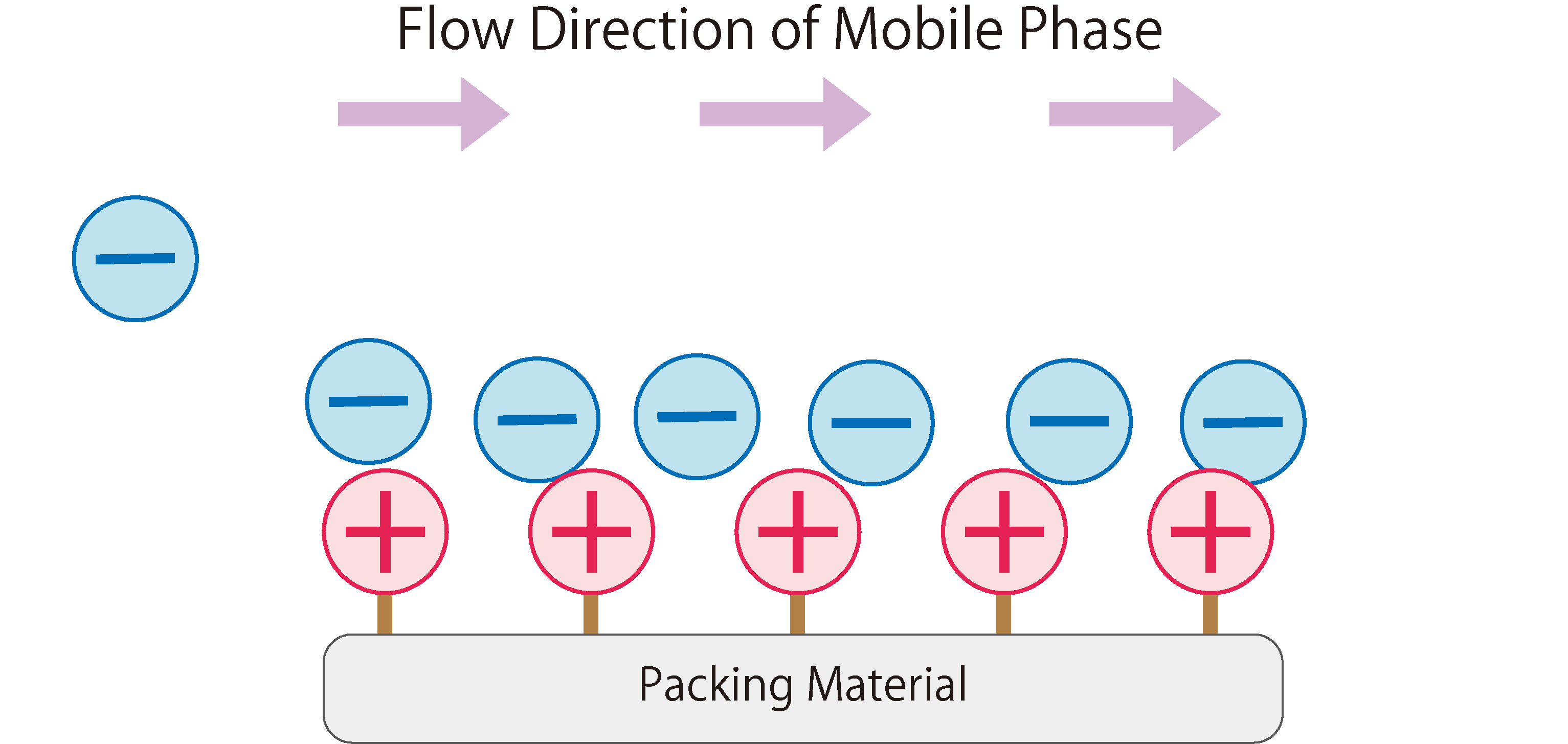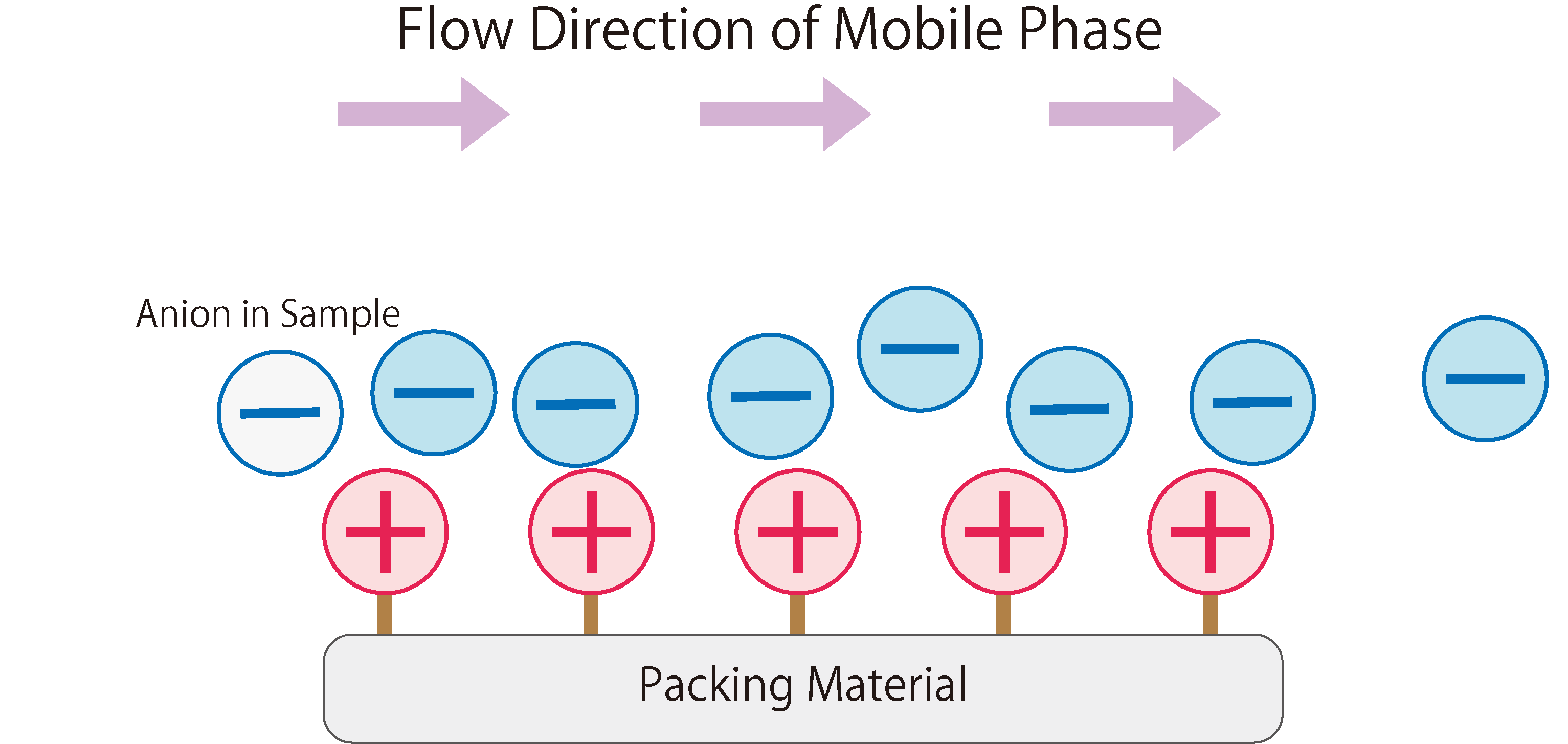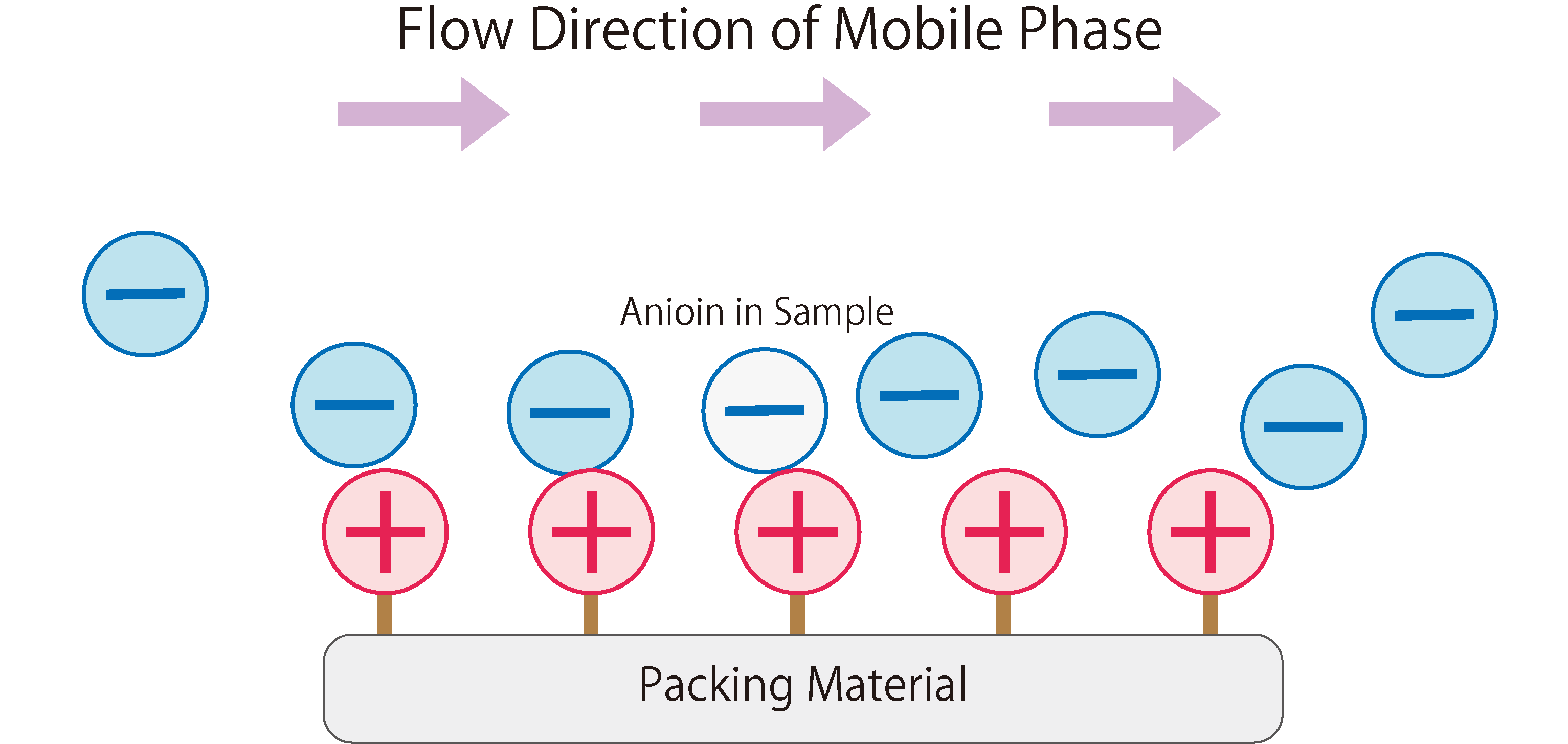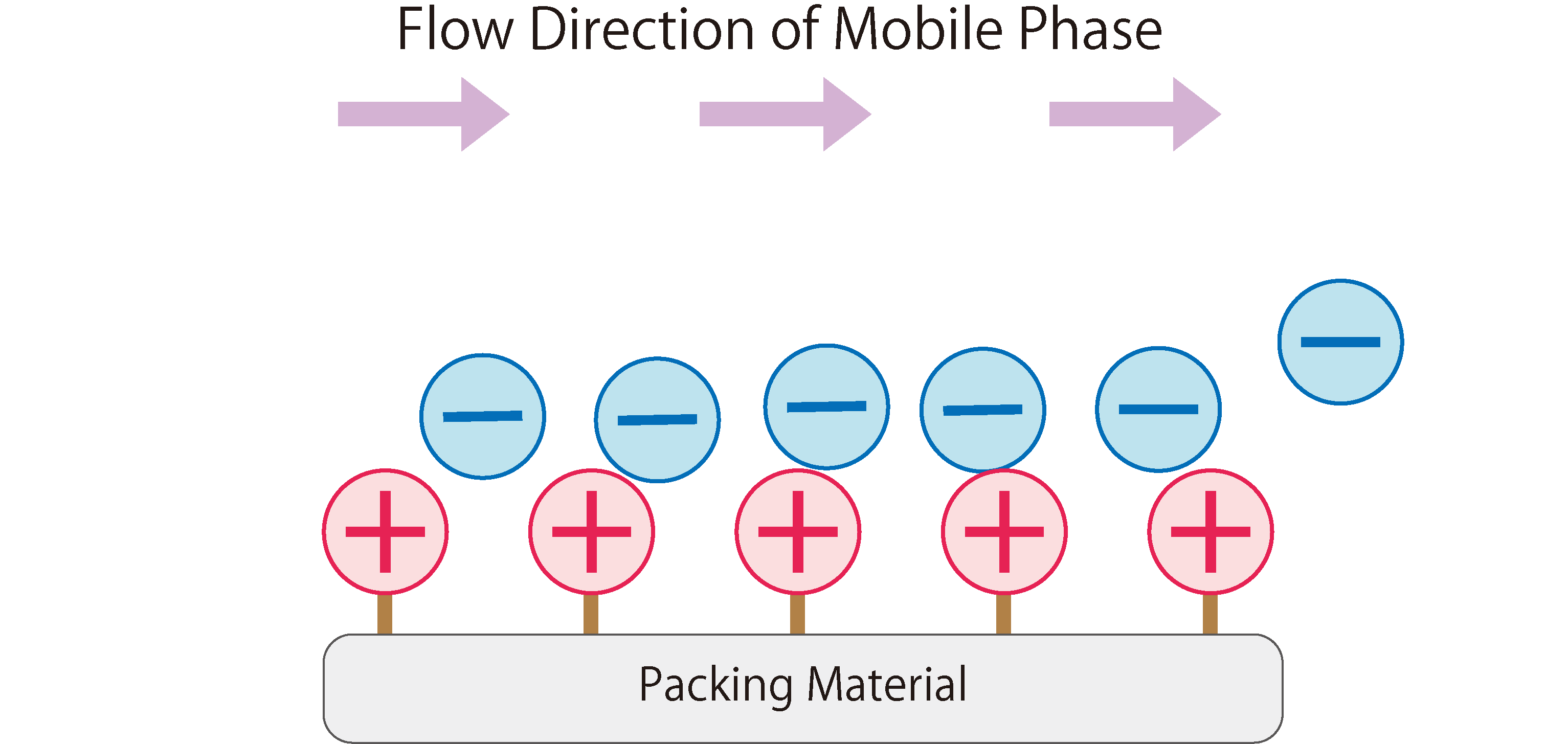
Fig. 1 The Workings of Anion Exchange Chromatography
Factors Affecting Separation
Different types of ions in the sample interact differently with the ion exchange groups, resulting in differences in the speed at which they move through the column. This difference is used to separate the ions in the sample. Ions with a smaller valence interact less with the ion exchange groups and therefore elute from the column faster. However, ions with the same valence or homologous elements will still elute faster if they have smaller ionic radiuses.
In ion exchange chromatography, compounds whose ionic state changes with pH can be further optimised by adjusting both the pH and salt concentration of the mobile phase, which can alter elution order.
Applications of Ion Exchange Chromatography
Inorganic Ion Analysis
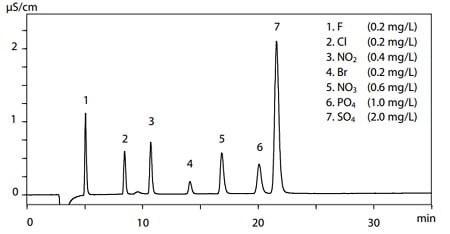
Fig. 2 Chromatogram of Anion Standard Solution
Figure 2 shows the chromatogram of standard solutions for seven anions. An anion exchange chromatography column (Shim-pack IC-SA3) was used in this analysis. F, Cl, and Br are halogen elements that eluted in order from small to large ionic radius, though their ionic values were the same. This demonstrates the high selectivity achievable through IEX for inorganic anion analysis.
Oligonucleotide Analysis
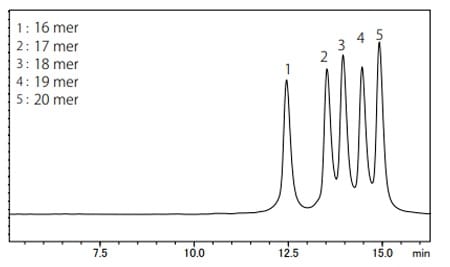
Fig. 3 Chromatogram of Oligonucleotides Mixture
Figure 3 shows the chromatogram of a five-sequence oligonucleotide mixture. An anion exchange chromatography column (Shim-pack BIO IEX Q-NP) was used in this analysis. The separation of oligonucleotides is based on the number of phosphate groups in the oligonucleotide, i.e., the difference in negative charges. Therefore, each oligonucleotide was separated by their length.
FAQ
How do I choose between anion and cation exchange chromatography?
The choice depends on the net charge of your target molecule at the working pH. Use cation exchange chromatography for positively charged analytes (such as basic proteins or metal cations) and anion exchange chromatography for negatively charged analytes (such as acidic proteins, nucleic acids, or inorganic anions).
What factors affect separation in ion exchange chromatography?
Separation efficiency is influenced by ionic strength, pH, flow rate, and column characteristics. Increasing salt concentration in the mobile phase competes with analyte ions for binding sites, promoting elution. pH affects the charge state of both the analyte and the stationary phase functional groups.
What is the difference between strong and weak ion exchangers?
Strong ion exchangers (such as sulfonic acid or quaternary ammonium) remain ionised across a wide pH range, providing consistent separation. Weak ion exchangers (such as carboxylic acid or tertiary amine) exhibit pH-dependent ionisation, offering more selective binding under specific conditions.
Related Products
-
-
Ion chromatography (IC) is used for analysis of inorganic and organic ions. It is categorized as suppressor IC and non-suppressor IC. Non-suppressor IC is composed of a conventional HPLC system combined with a conductivity detector, while suppressor IC requires an extra suppressor.
-
Shim-pack Bio IEX Columns are available in Q (quaternary ammonium) and SP (sulfopropyl) chemistries and are based on porous (Q and SP columns) and non-porous (Q-NP and SP-NP columns) hydrophilic polymers with low nonspecific adsorption.
In ion-exchange chromatography, if a sample contains compounds whose ionic state changes with pH, changing the pH of the mobile phase as well as the salt concentration of the mobile phase can change the elution order.
Learn More
Link










