Ion Pair Chromatography - Differentiating When to Use Alkyl Sulfonates and Perchlorates
Ion pair chromatography (IPC) is one technique used to separate charged substances. It is widely used to selectively analyze acids and bases, particularly with reverse phase chromatography. However, customers often complain that setting analytical conditions for IPC can be troublesome or good reproducibility is difficult to obtain. Such problems probably occur due to insufficient consideration given to selecting pair ions (ions with the opposite charge of target components) for adding to mobile phases, and to their use conditions. Therefore, the following explanation uses alkyl sulfonates and perchlorates as an example, which are commonly used in IPC of bases and cations, to provide an overview on how to determine which to use based on their respective characteristics.
Alkyl Sulfonate
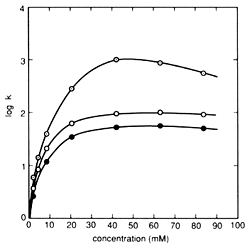
Fig. 1 Influence of Pair Ion Concentration on Component Retention Column: STR ODS-M, Mobile phase: 10 mM citrate (sodium) buffer solution containing sodium 1-octanesulfonate (pH 4.6) / acetonitrile solution = 5/1 (v/v), Flowrate: 1.0 mL/min, Column temperature: 40 °C, and Detection: 280 nm. Components: ○(Gray) - Dopamine, ○ - Epinephrine, ● - Norepinephrine
Alkyl sulfonate is a typical pair ion used in IPC of substances with a positive charge. Normally, alkyl sulfonate with 5 to 12 carbons is used as a sodium salt. In general, the separation mechanism used in reverse phase ion pair chromatography is explained as consisting of two processes - an ion pair distribution process, which pairs the target components with pair ions and captures them in a solid phase, and an ion exchange process, which retains the target components by ionic interaction with the pair ions hydrophobically adsorbed to the solid phase. However, in the case of alkyl sulfonate, the main mechanism is an ion exchange process. Therefore, the higher the number of carbons in the alkyl sulfonate, the stronger the retention effect on the component. In addition, for the same type of alkyl sulfonate, the lower the concentration of organic solvent in the mobile phase, the stronger the retention. (If an alkyl sulfonate with a large number carbons is used with an extremely low concentration of organic solvent, a pseudo-ion exchange mode occurs, where once equilibrium is reached, target components can be retained without adding pair ions to the mobile phase.) The pair ion concentration affects component retention as well, but surfactants like alkyl sulfonates exhibit a unique relationship between concentration and retention behavior. This relationship is shown in Figure 1. In areas where the concentration is relatively low, component retention increases in a linear manner, but once it becomes saturated at a given concentration (referred to as the "fold over point"), the retention level reverses direction and begins decreasing. This is explained by the alkyl sulfonate forming micelles, resulting in a secondary hydrophobic phase within the mobile phase. Therefore, there is a limited range of pair ion concentration that can be used for IPC.
Perchlorate
Unlike alkyl sulfonates, perchlorates (normally used as a sodium salt) are not themselves hydrophobic. Therefore, they cannot be used for ionic exchange purposes. Nevertheless, since perchlorates have a large ion radius, they readily form ion pairs and, consequently, can be considered completely in terms of the ion pair distribution process. Therefore, they have no fold over point in the relationship between concentration and component retention, so retention keeps gradually increasing as the concentration increases. Furthermore, the component retention effect is uniform, regardless of organic solvent concentration.
However, since the retention of components by perchlorates is based on the hydrophobic properties of the components themselves that appear when their charge is canceled by the formation of ion pairs, it may not be applicable for some components.
Differentiating the Use of Pair Ions
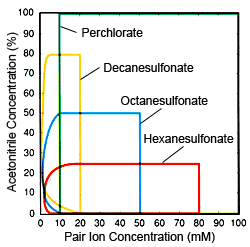
Fig. 2 Ion Concentrations and Organic Solvent (acetonitrile) Concentration Range Effective for Ion Pair Chromatography Red: Hexanesulfonate, Blue: Octanesulfonate, Yellow: Decanesulfonate, Green: Perchlorate
Based on the characteristics of respective pair ions, the following describes practical considerations for differentiating when to use different pair ions. The fundamental objective of using IPC and these pair ions is to increase the retention strength of bases and cations, but they are also used to suppress peak tailing (phenomenon common in ODS and other silica gel columns). For the first of these objectives, alkyl sulfonate is more useful, because it can control component retention over a wide range. For the second objective, perchlorates are preferable, because they are easier to use and can be used for any component. Therefore, in general, alkyl sulfonates are preferred for component retention, while perchlorates are preferred for suppressing tailing. However, if target components are hydrophobic ions, there is no significant difference between alkyl sulfonates and perchlorates. If an alkyl sulfonate is used, the concentration of the organic solvent in the mobile phase is extremely important when determining the number of carbons for the alkyl sulfonate. Eluting components at fixed positions using fixed organic solvent concentrations requires that the fewer the number of carbons, the higher the concentration. However, as shown in Figure 1, there is a limit to pair ion concentrations, so if the organic solvent concentration is high, alkyl sulfonates with few carbons cannot be used. However, that does not mean that higher carbon counts are necessarily preferable. Rather, fewer carbons is an advantage in terms of column equilibrium. The lower the organic solvent concentration and the lower the pair ion concentration, the longer it takes for the column to reach equilibrium, but if an alkyl sulfonate with high carbon count is used with a low organic solvent concentration, it will take a very long time for the column to stabilize because a low concentration is being used, of course. Consequently, it is important to decide the organic solvent concentration before selecting the number of carbons. Figure 2 shows general guidelines for determining use conditions (pair ion concentrations and organic solvent concentrations) effective for respective pair ions. Use it as a reference for selecting pair ions.
Setting Organic Solvent Concentrations
Decide the organic solvent concentration, by first considering the original hydrophobicity of the target components. In other words, consider how well the mobile phase retains the target components without including any pair ions. For example, if IPC is to be used to elute a component in 10 minutes, mobile phase conditions must allow eluting the component within 10 minutes without including any pair ions. Consequently, the lower limit for organic solvent concentration is the concentration that results in eluting the component in 10 minutes, so it is necessary to set the concentration equal to or higher than that. If IPC is being used to simply increase the retention strength of a component, any appropriate concentration equal to or higher than that could be selected, but if components with similar hydrophobicity must be separated, the concentration should be kept as close to the lower limit as possible, because the higher the organic solvent concentration, the smaller the relative retention. If target components include not only bases and cations, but also neutral substances or acids, the concentration that can adequately retain such components is considered the upper limit.






