Overview of Amyloid MS
Amyloid MS is a new blood analysis method developed by Shimadzu Corporation and the National Center for Geriatrics and Gerontology (NCGG) to screen for Alzheimer’s disease. The technology aims to predict early stage Alzheimer’s disease from a few drops of blood (about 0.5 mL).
Alzheimer’s Disease
Given the progressively aging populations in recent years, the number of dementia patients has been increasing. As of 2012, one in seven Japanese aged 65 or older had dementia, and the number is expected to increase further in the future. Measures to address dementia have become a social issue, with the Japanese Ministry of Health, Labour and Welfare and other relevant ministries initiating a Comprehensive Strategy to Accelerate Dementia Measures (New Orange Plan). Consequently, there is a need to establish methods for early detection, treatment, and prevention. Though a variety of diseases can cause dementia (such as Alzheimer’s dementia, cerebrovascular dementia, dementia with Lewy bodies, and frontotemporal dementia), Alzheimer’s dementia accounts for the highest number of patients, accounting for about 60 % of the total.
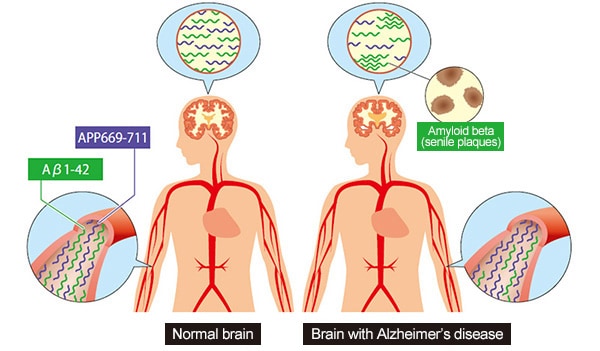
Though the causes of Alzheimer’s dementia have not been identified, several characteristic pathologies can be observed as the disease progresses. For example, amyloid beta accumulates on the surface of neuronal cells to form senile plaques, and tau proteins accumulate inside neuronalcells to change proteins to a lint-like form (neurofibrillary tangles).
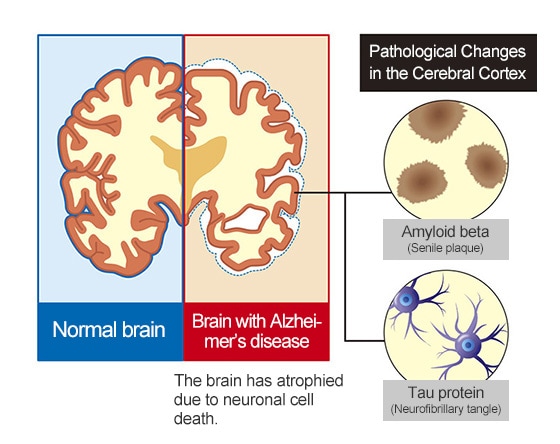
Therefore, one way to identify the pathologies of Alzheimer’s disease before the symptoms of cognitive decline appear is to visualize and numerically quantify amyloid beta and tau protein accumulation levels (quantities, concentrations, and distributions). For example, PET scans or cerebrospinal fluid (CSF) marker tests can be performed.
In addition, Shimadzu collaborated with the National Center for Geriatrics and Gerontology (NCGG) to discover biomarkers useful for predicting the quantity of amyloid beta plaques from a few drops of blood.
Road to Accurate Aβ-Related Peptide Detection
Even if high levels of amyloid beta plaque have accumulated, there are high concentrations of various extraneous substances in the blood, and only a very small quantity of amyloid beta is present. Therefore, it had been very difficult to detect amyloid beta in blood samples.
two main types of amyloid beta has been known to exist. One is “Aβ1-40,” a peptide consisting of 40 amino acids, and the other is “Aβ1-42,” which consists of 42 amino acids. Furthermore, at least ten other types with different lengths has been known to exist. The commonly used enzyme-linked immunosorbent assay (ELISA) lumps together multiple lengths of amyloid beta. Even if Aβ1-40 and Aβ1-42 types could be distinguished, ELISA cannot accurately measure other Aβ-related peptides (a generic term for amyloid beta of various lengths).
Given that mass spectrometers are well-suited to identifying and simultaneously measuring multiple types of substances, Shimadzu decided to try using a high-performance mass spectrometer system that resulted from participation in the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program) in 2010 to measure Aβ-related peptides.
Despite the technical difficulty of detecting small amounts of Aβ-related peptides with a mass spectrometer, that problem was overcome by developing a pretreatment method for blood. Moreover, biomarkers that correlate to the state of amyloid beta plaque were discovered during joint research work with NCGG, which led to successfully developing the world’s first technology that can predict amyloid beta accumulation using a blood sample.
-

Acquire blood

-

Pretreat blood

-
Analyze masses

-

Analyze Aβ-related peptides
Innovation 1—Blood Pretreatment Method
To accurately detect the quantities of each type of Aβ-related peptide, Shimadzu’s Naoki Kaneko tried a wide variety of blood pretreatment methods.
Eventually, he found a method that involves using a special antibody with an epitope for a sequence within Aβ-related peptides and performing two successive affinity purification steps.
By selecting an optimal surfactant for antigen-antibody reactions and using the optimal reagent for each step, including acidic solutions containing a surfactant for the first step and an organic solvent for the second step, using an appropriate quantity of solution for promoting contact with antibodies, and making other suitable adjustments, he discovered a method to extract only Aβ-related peptides from the blood and detect them with a sufficiently high S/N ratio using a mass spectrometer.
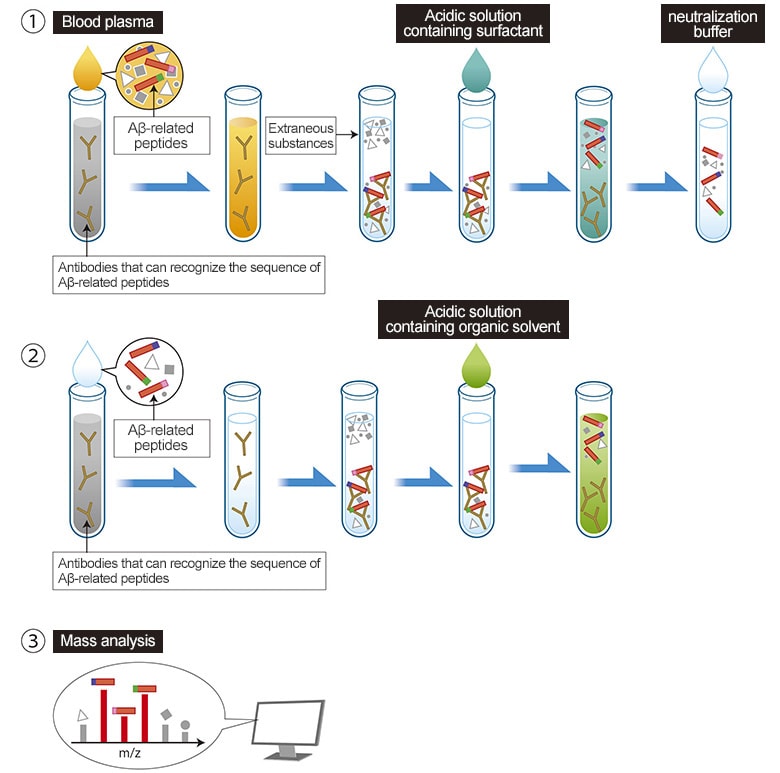
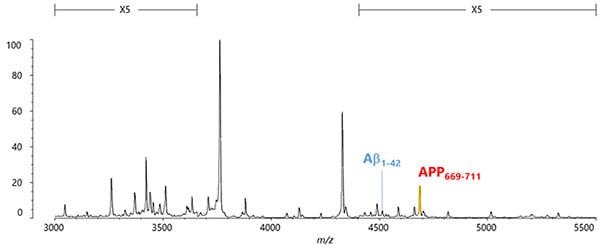
This method, which combines Shimadzu’s strengths in antibody technology and high performance mass spectrometry technology based on using a MALDI ion source*, can separate and measure 22 types of Aβ-related peptides. The 22 types even include eight new types that had never been confirmed to exist in blood before.
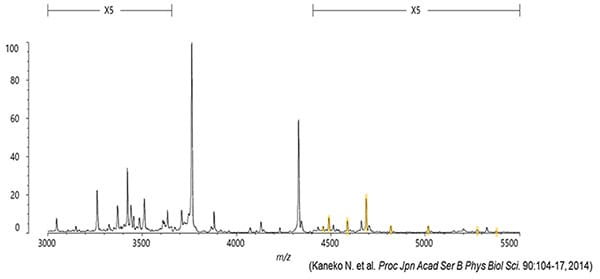
・ Patent No.: 06424757
・ Academic paper: Proc. Jpn. Acad., Ser. B (2014) 90, 104-17
2. Method for Evaluating Amyloid Beta Accumulation Levels (Biomarkers)
By using the blood pretreatment and accurate mass spectrometer system, 22 types of Aβ-related peptides, including eight newly discovered types, can be measured. So, how can the resulting data be used for the early detection of Alzheimer’s dementia?
To gain that knowledge, we visited the NCGG and started collaborative research with Dr. Katsuhiko Yanagisawa, who is a leading authority in Alzheimer’s research.
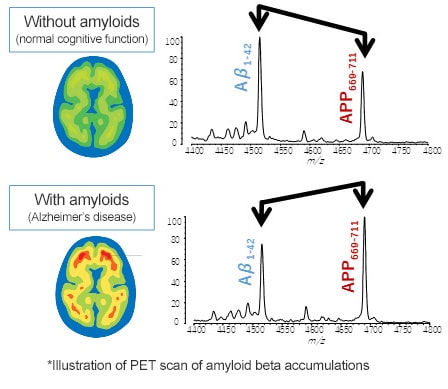
To discriminate between subjects with positive and negative results based on a clear criterion in clinical research, Dr. Yanagisawa proposed a biomarker research using Aβ-PET scan results rather than using the typical approach based on patient interviews.
One and a half years after the start of the collaborative research, we discovered the tendency for the quantity of the newly discovered peptide APP669-711 to be higher than Aβ1-42 as the Alzheimer’s disease progressed. In other words, that suggested that by using APP669-711/Aβ1-42 as a new biomarker, it might be possible to accurately discriminate between patients with and without Alzheimer’s pathologies (amyloid beta plaques).
・ Patent No.: 06410810
・ Academic paper: Proc Jpn Acad Ser B Phys Biol Sci. 2014, 90(9): 353-364
The reason the intensity ratio between Aβ1-42 and APP669-711 varies depending on the state of the amyloid beta accumulation is presumably due to the difference in aggregation ability between the two peptides.
Even in normal healthy individuals, some of the Aβ1-42 produced in the brain leaks into the cerebrospinal fluid or blood.
When amyloid beta starts to accumulate in the brain, the Aβ1-42 produced successively binds (aggregates) to the amyloid beta, which results in less leakage into the blood. In contrast, APP669-711 has a different amino acid sequence on each end, which significantly reduces the level of self-aggregation compared to Aβ1-42. Consequently, it is less likely to bind to amyloid beta, with presumably a fixed ratio leaking into the blood, regardless of the amyloid beta accumulation levels in the brain.
Also, the amount of Aβ1-42 leakage not only varies depending on the amyloid beta accumulation levels but is also affected by the individual’s overall condition. By calculating the ratio versus leakage of APP669-711 affected in the same way, the whole-body effects can be eliminated to isolate only the variations caused by the amyloid beta accumulation.
・ Academic paper: Nature (2018) doi:10.1038/nature25456
The technologies, products, and other information indicated are intended for research purposes.
For Research Use Only. Not for use in diagnostic procedures.
Co-Creation
The technology described above was established by combining Shimadzu’s expertise in mass spectrometry technology with knowledge from dementia research at the National Center for Geriatrics and Gerontology.
Link to National Center for Geriatrics and Gerontology website


