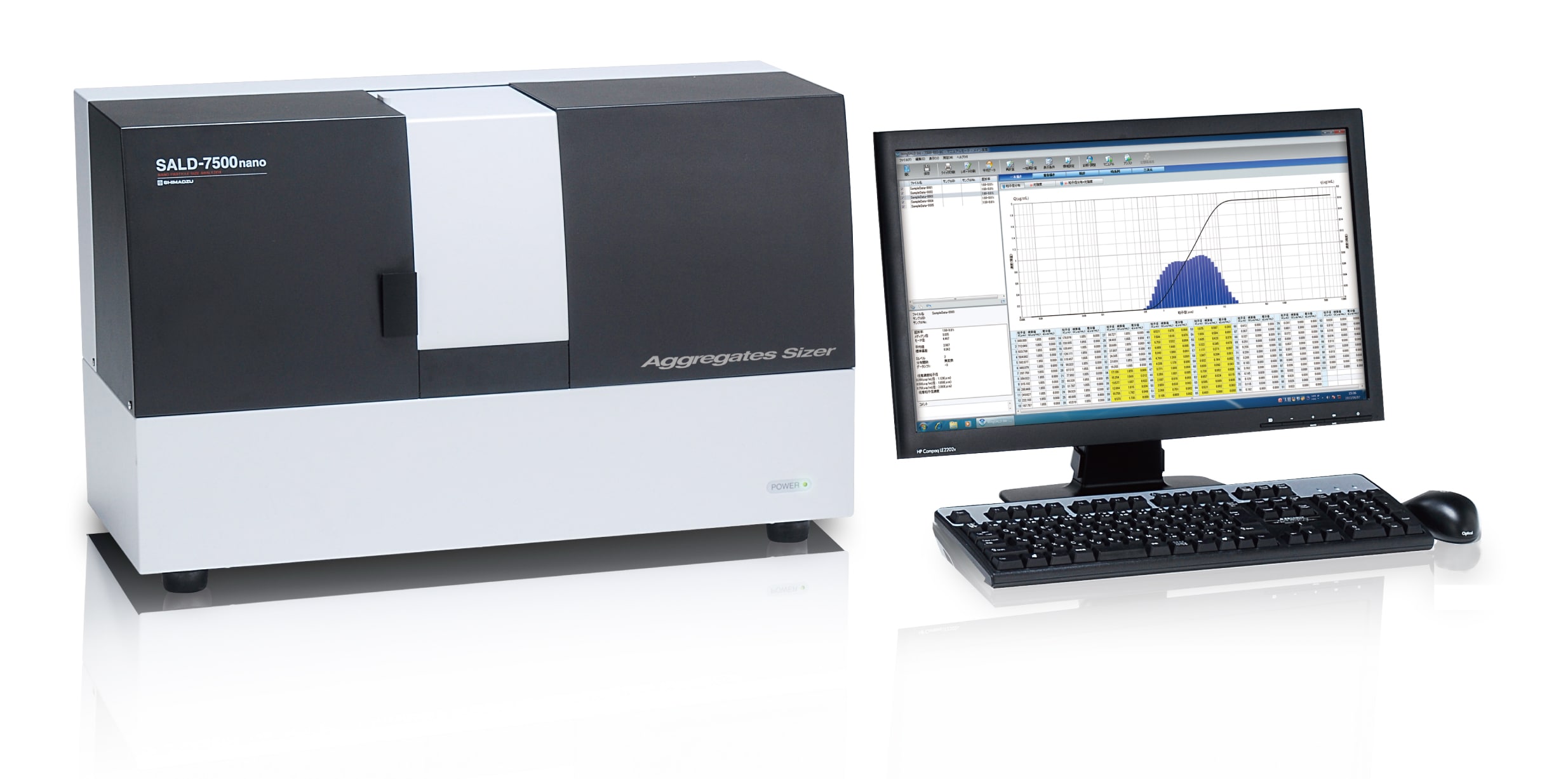
Aggregates Sizer
Powder Property Analys
Biopharmaceuticals contact a variety of materials during their production, storage, and transport that include metal, plastic and glass. Protein stability differs depending on the materials it comes into contact with. Although investigations must be performed into materials appropriate for contact with biopharmaceuticals, analyzing the many materials a biopharmaceutical contacts during processing increases costs. Furthermore, several months or more are needed to perform a long-term evaluations of storage stability. Accelerated stability testing of contact materials performed in advance would improve the efficiency of investigations into production processes for biopharmaceuticals. We conducted accelerated stability testing of proteins via the monitoring of aggregate formation during application of physical stress at constant temperature. Three agitator plate materials (PEEK, stainless steel, and glass) attached to the “Aggregates Sizer TC (with temperature control)“, Aggregation Analysis System for Biopharmaceuticals was used for testing. The results we obtained indicated the importance of temperature control for stability testing and suggested different materials have different effects on aggregation.
August 26, 2016 GMT
Some products may be updated to newer models