LabSolutions UV-Vis
Spectrophotometric Analysis
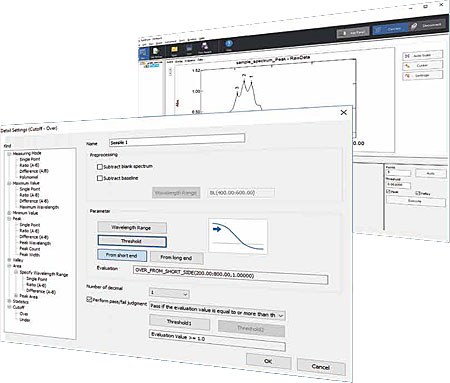
Ultraviolet-visible spectrophotometers are used routinely in various industries for acceptance inspections of raw materials and quality inspections of products. In the pharmaceutical industry in particular, testing compliant with the Pharmacopoeia is prescribed as the criteria to determine whether or not the properties and quality of pharmaceuticals are appropriate. However, the testing requires burdensome tasks, such as reading specific peaks or calculating the absorbance ratio of multiple peaks from the obtained data, to judge the acceptability of the substances. The LabSolutions UV-Vis software is equipped with the spectral evaluation function as a standard feature so that the tasks which generally require time and effort can be performed efficiently. This function automatically carries out analysis, including peak detection and calculation after spectrum measurement and makes a pass/fail judgment. This article introduces the details of the spectral evaluation function using an example analysis for identification of "rutin, for thin-layer chromatography", which is described in the Japanese Pharmacopoeia (JP).
June 24, 2019 GMT
Some products may be updated to newer models