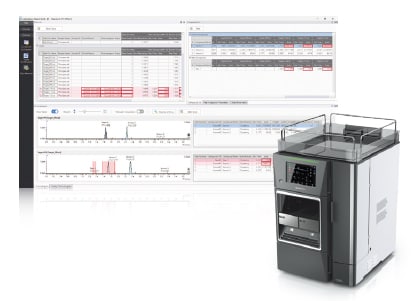
LabSolutions Detect
- LabSolutions Detect enhances the reliability and productivity of quality control processes while minimizing the risk of errors or oversights associated with manual visual inspection. - By overlaying chromatograms through alignment processing, the software enables rapid detection of abnormalities such as impurity contamination.
In quality control of pharmaceuticals and functional foods, verification of impurities and active ingredient content is essential to ensure product efficacy and safety. These analyses are generally performed by qualitative and quantitative LC methods, with relevant guidelines including ICH Q3A, Q3B1), and the GMP guidelines for functional foods scheduled to take effect in 20262). In such verification, numerous chromatograms must be examined to identify peaks based on retention times, compare quantitative results with acceptance criteria, and confirm the presence or absence of impurities. When performed manually, these extensive tasks present a risk of human error and missed detections. LabSolutions Detect, an anomaly peak detection support software, addresses this issue by automatically analyzing differences between reference chromatograms representing impurity-free conditions (reference data) and chromatograms obtained in routine quality control (target data). This approach minimizes the risk of overlooking variations in active ingredient content or impurity contamination. As a result, LabSolutions Detect enables efficient, regulatory-compliant quality control operations. In this article, a case example of streamlining quality control processes is presented using a model sample of pramipexole hydrochloride, a compound listed in the USP.
September 29, 2025 GMT
Some products may be updated to newer models