USP-Compliant Analysis of Vitamins in Dietary Supplements Analysis of Calcium Pantothenate by Nexera™ XR
The United States Pharmacopeia (USP) provides standards for quality control of dietary supplements and specifies testing methods and judgment standards for dietary supplements. Because many dietary supplements are distributed globally, USP compliance has become an important judgment standard for verifying the quality of upplements for consumers.
The substance that was analyzed here is pantothenic acid (pantothenate), which is a water soluble vitamin. Also called vitamin B5, pantothenic acid is a substance in which β-alanine is bonded with pantoic acid. Many dietary supplements contain pantothenate in the form of its calcium salt.
“Oil and Water Soluble Vitamins with Mineral Tablets – Calcium Pantothenate” of USP40-NF35 describes two analysis methods using HPLC and one microorganism quantitation method. In the HPLC method in “Method 3,” calcium pantothenate is detected with an ultraviolet-visible (UV-VIS) absorbance detector after separation with a reversed-phase ODS column.
This article introduces an example in which the calcium pantothenate in a dietary supplement was analyzed using a Nexera XR, which is part of the Shimadzu Nexera Series of ultra high performance liquid chromatographs. An analysis was also conducted with a Prominence™ Series HPLC, confirming that the same results can be obtained with that system, as reported here.
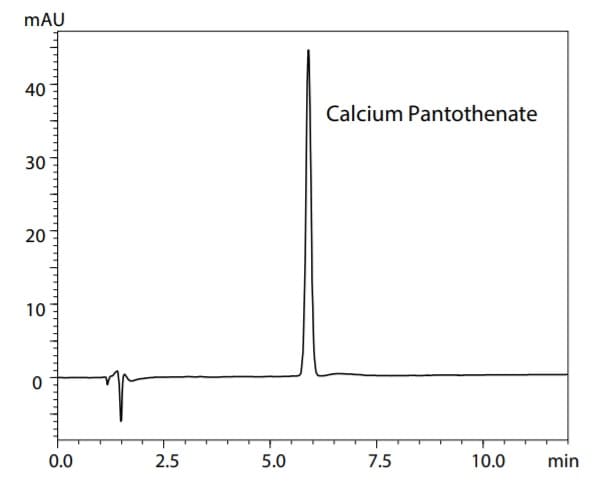
Chromatogram of Calcium Pantothenate Standard Solution (40 mg/L)
Related Products : Nexera series


