Method Development System - Applications
Automatic Optimization of Gradient Conditions with AI Algorithm

Most of the documents on the LITERATURE is available in PDF format. You will need Adobe Acrobat Reader to open and read PDF documents. If you do not already have Acrobat Reader, you can download it free at the Adobe's Website. Click the GET ADOBE READER icon on the left to download a free copy of Adobe Acrobat Reader.
Nucleic acid drugs, such as antisense oligonucleotides, exert their effect by interacting with targets (genes and proteins) inside and outside of cells. Nucleic acid drugs are produced through chemical synthesis, but the synthesis process can introduce impurities such as shorter and longer length of products and protection groups. Therefore, proper separation of the target oligonucleotide is required.
Efficient Method Development of Monoclonal Antibody Size Variants by Size Exclusion Chromatography
Antibody drugs using monoclonal antibodies (mAbs) pose concerns about forming aggregates during production and storage in terms of their impact on safety and efficacy. ICH-Q6B requires the separation of impurities such as aggregates and fragments in antibody drugs and to determine their content. Therefore, monitoring these impurities by size-exclusion chromatography (SEC) is one of the most important analyses during the production of mAb.
Efficient Method Development on Pharmaceutical Impurities Using Single Quadrupole Mass Spectrometer
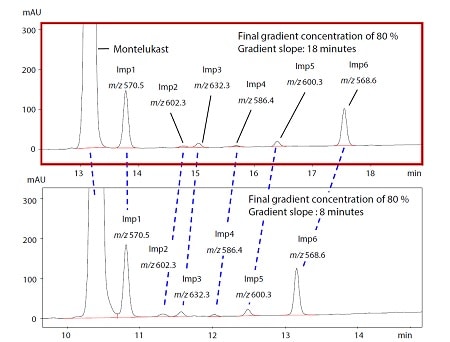
This article introduces the development of a robust LC method for impurities on Montelukast. By changing each parameter of gradient program, the resolution of Montelukast and each impurity was evaluated by visualizing through design space. Though it was previously difficult to accurately track each impurity with similar UV spectra using photodiode array detector (PDA), LCMS-2050 can solve this problem. Furthermore, by utilizing design space of resolution and RT of last eluting peak, it is possible to efficiently develop method that provides both excellent resolution and shorter analysis time.
Efficient Method Development through Design Space Evaluation on Different Brand of Columns
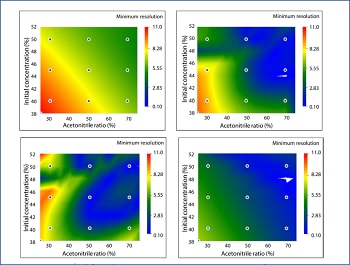
This article introduces an example of column screening using the design space evaluation concept. The selectivity of six brands of C18 column was visualized by design spaces considering different mobile phases composition and different gradient program. This makes it possible to understand the impact of the parameters on separation, enabling to search for the best column with a smaller number of analyses. In this article, it was found that the Shimadzu Shim-pack Arata C18 column has unique selectivity in comparison with the other C18 columns.
Efficient Method Development on Pharmaceutical Impurities based on Analytical Quality by Design
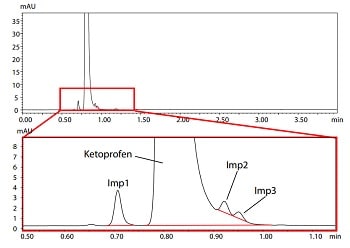
Since pharmaceutical impurities requires strict control to ensure safety, development of highly reliable analysis methods is necessary. This article introduces an example of its use in optimization and robustness evaluation of the column and mobile phase selected in the initial screening in order to realize high efficiency in the development of a robust LC method for impurities on ketoprofen. The resolution of each compound was evaluated by visualizing a "design space" after changing mobile phase composition, oven temperature and flow rate. In the step of robustness evaluation following optimization, resolution of each lot of columns was visualized and compared by design space to efficiently evaluate robustness among different lot of columns.
Efficient method development based on Analytical Quality by Design with LabSolutions MD software
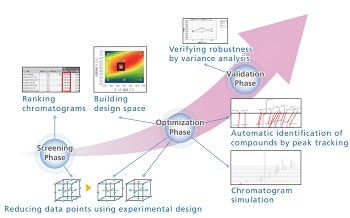
This article describes the ef¬ficient development of robust analytical methods based on Analytical Quality by Design (AQbD) using LabSolutions MD with small-molecule drugs. Method development based on AQbD consists of 3 phases including screening, optimization ,and validation. LabSolutions MD allows ef¬ficient method development by supporting every phase with dedicated functions such as experimental design, building of design space by automatic Peak Tracking function, and robustness evaluation.