Spectrophotometer Workstation Series - Features
To comply to ER/ES regulations
Solutions Delivered by the LabSolutions Series
LabSolutions resolves a number of problems commonly faced in the laboratory !
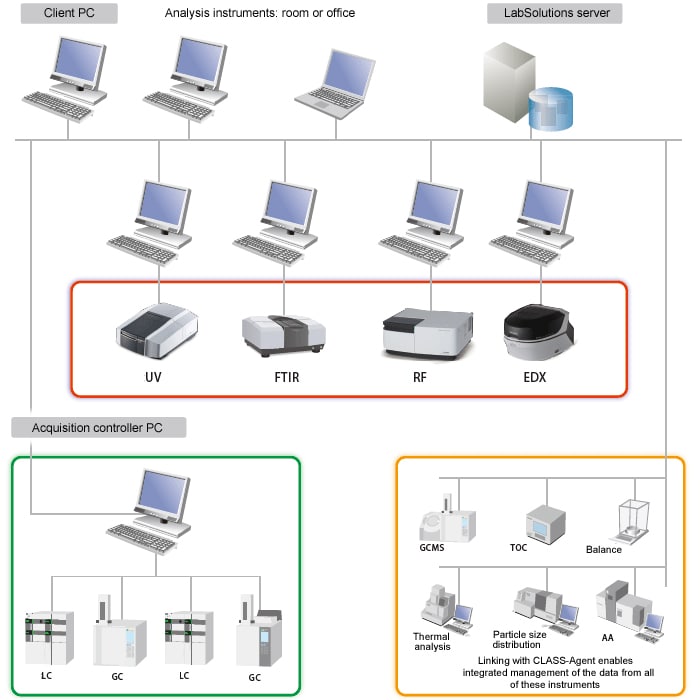
Analyze data from any computer*
LabSolutions CS control software allows access to the data on the network freely and independent of location while maintaining security. For example, pre-analysis operations can be performed from a computer terminal within the analytical laboratory. After the analytical run is initiated, data analysis performed from a computer located outside the laboratory. This operational format increases efficiency during analytical activities such as report writing.
Prevents mistakes in database management
LabSolutions DB and LabSolutions CS employ a database that ensures the secure management of analytical data. When used for the management of analytical data, this database prevents such mistakes as overwritten or deleted analytical data. This database also manages postrun analyses of analytical data by automatically attributing revision/version numbers to separate analyses, so analytical data is not overwritten. Old data can also be viewed with ease.
Firm security
Functions for setting an audit trail to ensure data reliability and for e-mailing events occurring on the system can be set. Various settings, such as setting the length, expiration date and complexity of passwords for user accounts, setting the lockout function to prevent illegal access, and registering settings for the deletion and alteration of registered users, can be made to enable highly secure system operation. Settings for overwriting data files and other information and settings relating to items to output in reports are also supported.
Information management on a project-by-project basis
LabSolutions DB and LabSolutions CS are designed with project management functions that allow data to be managed for each ask or system application. These functions include the ability to configure equipment control, user administration, security policy, and other data processing settings on a project-specific basis, making data search and operational management activities smoother between projects.
In addition to data, all user and other system information is managed centrally on a server.
In situations where user information is managed on each individual computer terminal, increasing the number of computers increases the burden on the person responsible for managing the information. With LabSolutions CS, all user information is managed centrally on the server, reducing the burden of individual computer terminal management and making the terminal administrator's job easier. Backing up data is also important. Because LabSolutions CS manages all data centrally on a server, no data is kept on networked computer terminals. The data stored centrally on the server can be backed up onto DVDs or other storage media, allowing this data to be referenced directly and eliminating the need to carry out a complete restore process.
Total, regulation-compliant support:Document preparation assistance
Building a system requires the documented formulation of management and operational management procedures, and that the system be operated according to those procedures. The service and work provided by Shimadzu for its customers goes beyond that normally expected of a supplier. Shimadzu assists in the preparation of documents necessitated by various industry regulations, and provides total, comprehensive support over the entire lifecycle of its systems. This begins with deliberations conducted prior to the installation of a system, and stretches to inspections that follow system installation as well as system updates. Shimadzu also reacts quickly to domestic and foreign trends in new requirements of regulatory authorities and the like. Shimadzu has a complete system of support in a state of continued readiness to meet the demands of its customers.
Applications of The Drug Development Processes
Component Analysis Is Possible Without Pretreatment of Powder Samples
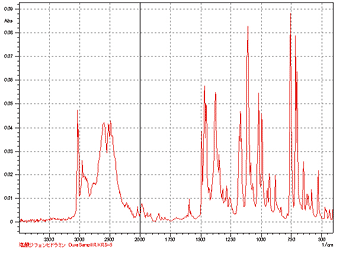
Use of an optional single-reflection ATR diamond prism eliminates necessity of pretreatment, even for powder samples. Measuring the infrared spectrum of a sample simply requires pressing it against the prism. Even for hydrochlorides, such as diphenylhydramine hydrochloride, measurement of the infrared spectrum can be made without taking into account ion exchange with the matrix, which might occur when using the pellet method.
Purity Tests in Accordance with the Japanese Pharmacopoeia
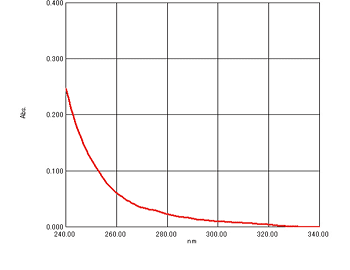
Measurement of ethanol
In the Japanese Pharmacopoeia, ultraviolet-visible spectrophotometry is specified as a purity test method for ethanol. It states that the absorbance at 240 nm, between 250 nm and 260 nm, and between 270 nm and 340 nm, not be more than 0.40, 0.30, and 0.10, respectively, if a cell with a 1-cm optical path length is used.
The regulations further state that the absorption spectrum determined with a 5-cm cell using water as a blank shows a flat absorption curve between 235 nm and 340 nm. A sample measurement of ethanol using a cell with a 1-cm optical path length is shown here.
Measuring Duloxetine Hydrochloride (USP)
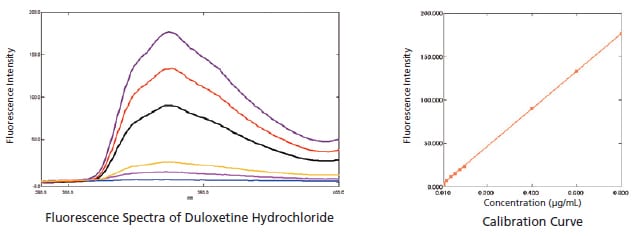
Duloxetine hydrochloride is a compound used as an antidepressant. In this example, an RF-6000 was used to measure duloxetine hydrochloride. The results indicated a lower limit of quantitation of 0.0007 μg/mL and a lower limit of detection of 0.0002 μg/mL, showing the ability of the RF-6000 to measure very low concentrations.


