Spectrophotometer Workstation Series
To comply to ER/ES regulations

Shimadzu's spectroscopy workstation series complies with ER/ES regulations (such as FDA 21 CFR Part11), enabling safe, reliable management of data.
- As a part of the LabSolutions family of control software, advanced security and user administration features are provided.
- Complies with ER/ES regulations including FDA 21 CFR Part 11 and PIC/S GMP.
- Centralized management of spectroscopy data (UV, FTIR, RF and EDX), as well as LC and GC data, on a networked server allows simple control over security and backups.
- Terminal Services can be used to control the Spectrophotometer application from a remote location, even when the application is not installed on the client PC.
Features
-
LabSolutions resolves a number of problems commonly faced in the laboratory !
-
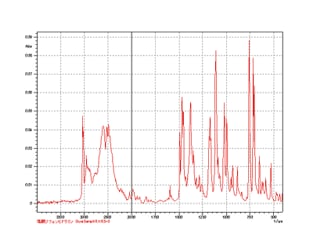
Component Analysis Is Possible Without Pretreatment of Powder Samples
Measurement of Diphenylhydramine Hydrochloride Use of an optional...
News / Events
-
Particle Analysis System for Microplastics has been released
This system can quickly calculate the number of particles, area, volume, mass, and individual particle qualities of microplastics based on the measurement results from an infrared microscope or an infrared Raman microscope.
-
UV-Vis Spectrophotometer UV-2600i Plus/UV-2700i Plus has been released
Accommodating a wide range of accessories, UV-2600i Plus/UV-2700i Plus excels in diverse applications, measures slight absorbance differences, and ensures robust data management and compliance.
-
UV-Vis Spectrophotometer UV-1900i Plus has been released
Experience unmatched precision and ease with UV-1900i Plus. Featuring a refined user-friendly interface, ultra high-speed scanning, and comprehensive support functions, UV-1900i Plus ensures accurate and efficient measurements for all your needs.
-
TEC MCT (Peltier Cooled MCT) Detector is now available
Equipping the AIMsight Infrared Microscope or the AIRsight Infrared/Raman Microscope with the TEC MCT (peltier cooled MCT) detector makes it possible to obtain infrared spectra without using liquid nitrogen.
-
FTIR TALK LETTER Vol. 43 has been published
The article about the Introduction to the Spectrum Advisor can be used on all Shimadzu FTIR systems controlled by LabSolutions IR. Learn the key points of infrared spectral analysis for aliphatic unsaturated hydrocarbons and aromatics.
-
Analytical Solutions for Microplastics
Shimadzu provides analytical and measuring instruments for the study of a variety of plastic materials: for R&D, characteristic evaluation of raw materials, quality control for plastic products, and deterioration analysis. With these diverse techniques, Shimadzu provides optimal solutions for microplastics research.