Inorganic Anion Detection
The concentration of inorganic anions is controlled or regulated in a wide variety of fields, such as public water systems, river water, various types of drinking water, and industrial effluents. This page discusses detection methods for analyzing these inorganic anions.
Electric Conductivity Detection
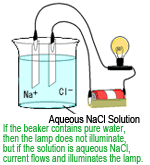
Inorganic ions are commonly analyzed using ion chromatographs, with the typical detection method being electric conductivity detection, which is based on detecting the electric conductivity of ions.
When voltage is applied to a pair of parallel plate electrodes in an aqueous solution, a current flows, if ions are present, between the electrodes. The electric conductivity can be determined by measuring that current.
If there are ions from the sample contained in the mobile phase flowing from the separation column outlet to the detector, then the electric conductivity in that area will vary according to the variation in ion concentration. The detector used to detect such variations is an electric conductivity detector. This detects all ions existing in aqueous solutions.
Each ion has a characteristic constant called the equivalent conductivity, where the larger this value, the larger the detected peak. It is expressed in terms of S-cm2/mol units and indicates how easily current flows per mole.
There are two types of electric conductivity detection, non-suppressor and suppressor methods. The following describes these detection methods for an example of anion analysis.
1) Non-Suppressor Method
This method connects the electric conductivity detector directly to the outlet of the anion separation column.
Anions in the sample are retained in the separation column by anion exchange to separate them from cations in the eluent. Due to the ion exchange, the concentration of anions in the eluent in this region decreases.
Electric conductivity detectors detect the total of all three types of ions - anions in the sample, anions in the eluent, and cations in the eluent (Figure 1a). Therefore, a low concentration aqueous organic acid solution with high elution strength is used as the eluent.
If an electric conductivity detector is available, this method enables using a normal HPLC system for inorganic anion analysis.
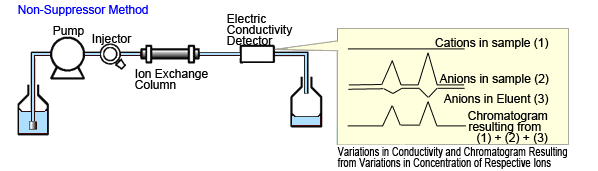
Fig. 1a Non-Suppressor Method
(2) Suppressor Method
The suppressor method changes the eluent composition to a composition with lower electrical conductivity.
Solutions such as aqueous sodium carbonate are used as the eluent. When anions are separated, the presence of sodium ions generates eluted carbonate ions. Eliminating the sodium ions just before the detector changes the eluent to a slightly acidic aqueous carbonate solution, with lower electric conductivity.
Meanwhile, in the regions where the inorganic anions in the sample elute, the carbon dissociation equilibrium shifts toward creating more H+ ions that become pair ions in order to maintain balanced charges (see the chromatogram of H+ ions in the eluent in Figure 1b). Because hydrogen ions have a higher equivalent electric conductivity than other ions, this increases the peak response (Figure 1b).
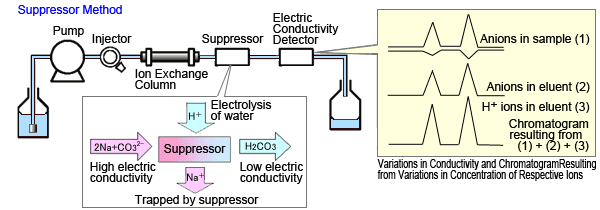
Fig. 1b Suppressor Method
Thus, the suppressor mode anion analysis enhances sensitivity by (1) reducing background levels and (2) increasing the peak response.
For cation analysis, the suppressor method results in almost no increase in peak response because the equivalent conductivity of the pair ions (OH-) in the eluent is lower than the equivalent conductivity of the hydrogen ions (H+). Additional Information: Peak Response Using Suppressor Mode for Cation Analysis
When to Use the Non-Suppressor or Suppressor Modes
Ion chromatographs not only can be used to detect inorganic ions, but also other ions as well, such as organic acids and ions with differing hydrophobicity. Because they can be used to detect a variety of substances, it is necessary to specify separation conditions that avoid overlapping peaks for target components and contaminant components. Since non-suppressor and suppressor modes use mobile phases with different pH levels, which may have a significant effect on ion separation.
Because the suppressor method uses sodium carbonate or sodium hydrate as eluents, it separates ions under basic conditions at pH levels of 7 or higher. Then, when the eluent passes through the suppressor, these changes in composition to aqueous carbonic acid or waterthe ions are detected under slightly acidic to neutral conditions. In contrast, the non-suppressor method generally separates ions under slightly acidic to neutral conditions, and then the eluent passes through the detector with the same unaltered composition. This means detected ions remain in the same eluent composition from separation to detection.
Because the separation and detection principles differ in this way for non-suppressor and suppressor methods, the eluents have significantly different pH levels. For that reason, they can result in completely different separation patterns, even when analyzing the same sample.
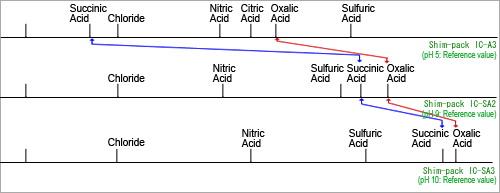
Fig. 2 Eluent pH and Elution of Organic Acids
Figure 2 shows the relationship between eluent pH and the elution position of organic acids. Due to the significantly different pH level of eluents used for non-suppressor (Figure 2 - upper) and suppressor (Figure 2 - middle and lower) methods, this figure shows how the dissociation level of organic acids from separation varies significantly as well, which changes the elution position.
When separation from contaminants is difficult using one detection method, the other detection method may allow separation with no problems at all. Therefore, one of these two detection method can be used as an alternative to the other in analysis.
Reference: Product Information Ion Chromatographs
Direct Absorbance Detection
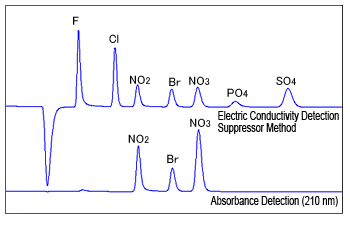
Fig. 3 Example of Electric Conductivity Detection and Direct Absorbance Detection
Some inorganic anions have absorption peaks in the UV wavelength range. Nitrite, bromide, nitrate, and other ions can be detected at wavelengths near 210 nm, whereas chloride and other ions can be detected near 190 nm.
However, organic acid type eluents used for the non-suppressor method cannot be used because the eluent itself has absorption peaks near 210 nm. Typically, direct absorbance detection also uses low-absorption carbonic acid based eluents which is used for the suppressor method.
Indirect Absorbance Detection
This method is based on the change in absorbance that occurs when an eluent with high absorbance is used to elute inorganic anions with low absorbance. The absorbance is changed when ions exchange occurs between the UV-absorbent ions added to the eluent and anions in the sample and change their concentration. Therefore, the composition of the eluent used must have a low concentration, high elution strength, and high molar extinction coefficient. Since the change in absorbance is negative, the chromatogram obtained has negative peaks. Also, since the peak size is proportional to the sample concentration, the lower the equivalent electric conductivity, the higher the relative increase in sensitivity that can be expected.
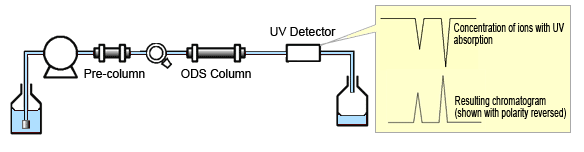
Fig. 4 Indirect Absorbance Detection
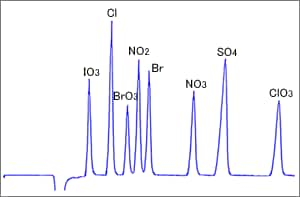
Fig. 5 Example of Indirect Absorbance Detection
Figure 5 shows an example of indirect absorbance detection using a reversed-phase column (ODS column). Inorganic anions are separated since the ion pair reagent added to the eluent functions as a pseudo ion exchange group on the ODS surfaces. The detection wavelength is decided based on the absorption wavelength of the organic acid added to the eluent for detection. This organic acid functions as an elution ion for separating anions. (Ie)
Additional Remarks Regarding Indirect Absorbance Detection
- Adding grade 4 ammonium or amine-based reagents tends to cause high pH levels in the vicinity of the ODS stationary phase, which could cause the ODS column to deteriorate. To protect the analytical column, we recommend using a GRD-ODS or other pre-column connected between the pump and injector.
- System peaks form the mobile phase reagent can appear after inorganic ions have eluted.
- In spite of lower sensitivity than electric conductivity detection, indirect absorbance detection has an advantage of being able to analyze inorganic ions even in a situation where only reversed-phase chromatograph can be used (cases where only an ODS column and UV detector are available).
Additional Information: Peak Response Using Suppressor Mode for Cation Analysis
Figure 6 shows the change in conductivity and resulting chromatograms corresponding to changes in concentrations of respective ions, when using non-suppressor and suppressor methods for cation analysis.
For the non-suppressor method, the chromatogram was obtained by adding the electric conductivity of (2) the cations in the sample and (3) the cations in the eluent. The equivalent conductivities of Na, NH4, K, Mg, and Ca cations in the sample are approximately ranged from 50 to 70 Scm2/mol, whereas the hydrogen cations in the eluent (H+) have an equivalent conductivity of 350 Scm2/mol, so the difference equivalent conductivity is 280 to 300 Scm2/mol.
For the suppressor method, the chromatogram was obtained by adding the electric conductivity of (4) the cations in the sample and (5) the anions in the eluent. Since the equivalent conductivity of the hydroxide anions (OH-) in the eluent is 200 Scm2/mol, the total equivalent conductivity is 250 to 270 Scm2/mol.
Consequently, considering the contribution to peak height by hydrogen ions (cations in the eluent) when using the non-suppressor method and the contribution to peak height by hydroxide ions (anions in the eluent) when using the suppressor method, switching to the suppressor method for cation analysis would not improve peak response.
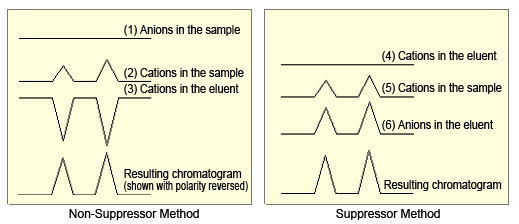
Fig. 6 Change in Conductivity for Respective Ions and Resulting Chromatograms


