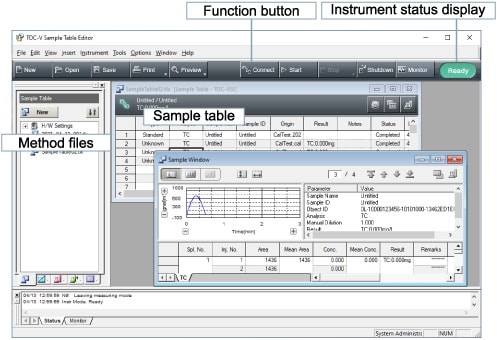
TOC-Control V
TOC-Control Software
A wealth of functionality
Useful functions facilitate your analysis operations.
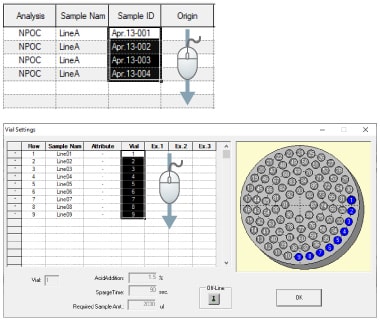
The character strings, serial numbers, and vial numbers can be batch entered in series by dragging over the cells.
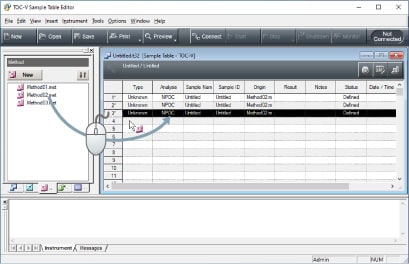
Addition of samples to serial measurements already in progress
Samples can be added during serial measurements using an autosampler.
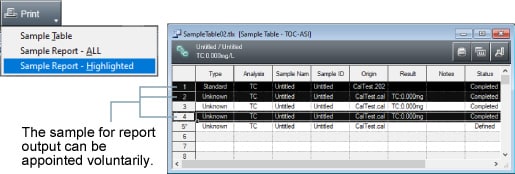
Selection of samples for report output
Use [Shift] and [Ctrl] keys to select desired samples to add to batch reports for all samples in a table. The reports can then be output.
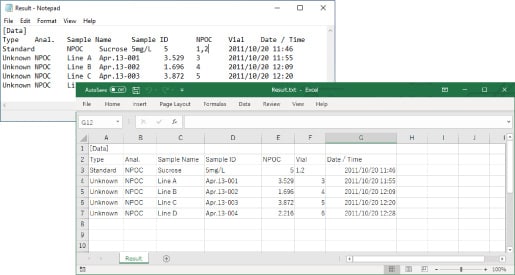
Import or export of text files
Measurement results can be exported as text files. These files can then be imported by Excel or other applications. Similarly, text files created according to a specified format can be imported as measurement schedules.
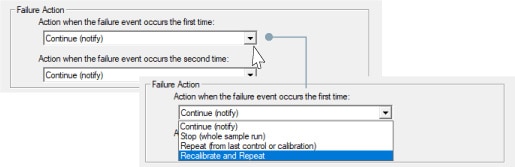
Quality control
Quality control samples can be inserted in measurement schedules. If measurement results exceed a pre-specified range, recalibration or other procedures can be performed automatically.
Features to ensure data reliability
Data Reliability
In environmental fields as well as pharmaceuticals and food production, a variety of regulations and guidelines have been implemented in order to ensure the reliability of measurement data. (In the pharmaceutical industry, these involve GLP/GMP and US FDA 21 CFR Part 11 compliance, while laboratories and calibration agencies are bound by ISO17025 etc.) These regulations target a broad spectrum of areas including experimentation and analysis methodology as well as formulation of inspection and calibration methods for experimental conditions and the instruments used, and the training of required staff. The handling of data management by digital media, an area that has grown explosively in recently years, is also referenced.
Security and integrity are maintained by data system management using TOC-Control V, and the LabSolutions network-compatible analysis data management tool. This protects valuable customer data, and reinforces compatibility with electronic records/electronic signature regulations. (ER/ES: electronic records, electronic signatures.)
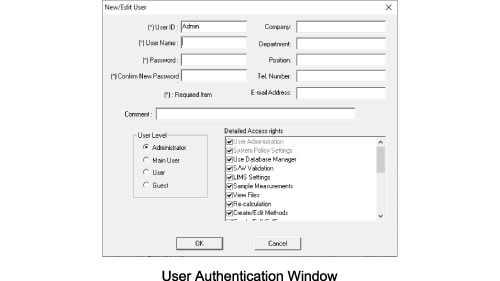
Security function settings
With the TOC-Control V, security can be configured to suit the operation environment. Security related configurations can be set during installation to deal with issues such as user authentication via ID/password, logs of operating history, and prohibitions on deletion or editing of measured data.
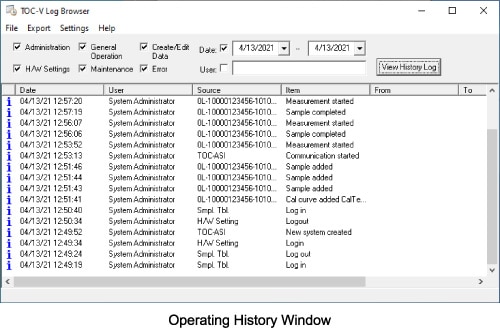
Operating History
A record of operations performed by the TOC-Control V software and measuring instruments can be maintained automatically. In addition to the dates and user names associated with operations, the details of settings changes can be tracked, as well as instrument operating status.
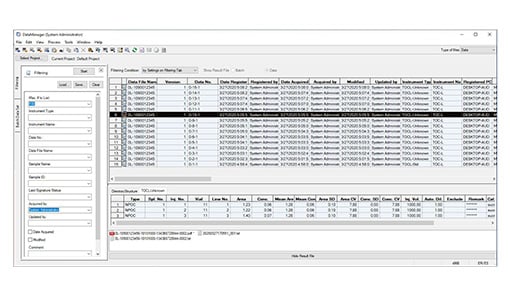
Link with LabSolutions
When performing measurements, both measurement data and meta data (including calibration curve information, methods, and instrument settings) can be integrated and exported as a data profile. This information can automatically be exported to databases managed by LabSolutions, an analysis data management support tool compatible with the Shimadzu network. This makes it possible to integrate management of this data with data from other Shimadzu analysis instruments.
News / Events
-
TOC-1000e S has been released
TOC-1000e S is an online TOC analyzer for ultrapure water in semiconductor manufacturing, detecting hard-to-decompose organics like urea with high precision to improve yield.
-
The TOC 50th anniversary special website has been opened.
The website has been established to explain the basic knowledge about TOC and describe the history of Shimadzu TOC analyzers.