Please explain the principles, advantages, and disadvantages of EI
1. BASIC MASS SPECTROMETRY
Please explain the principles, advantages, and disadvantages of EI.
EI (Electron Impact)
EI is the fundamental ionization mode. When problems are encountered with EI, other ionization methods are used.
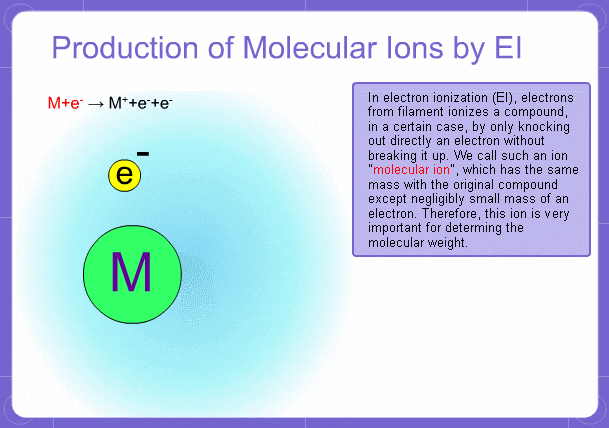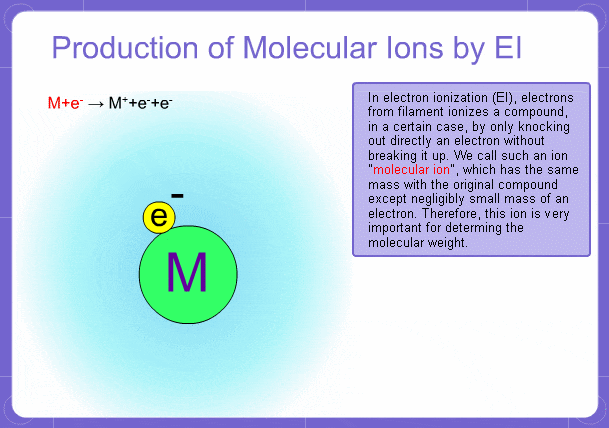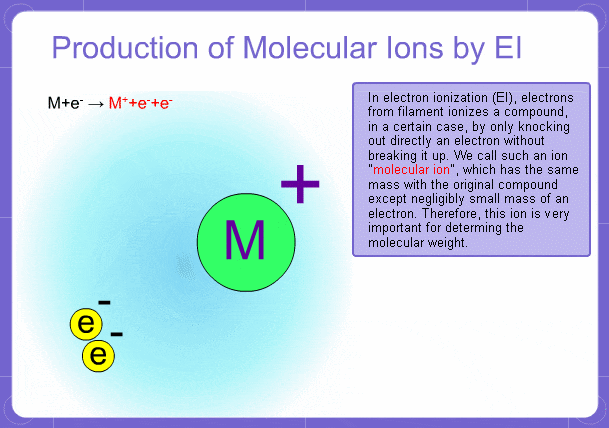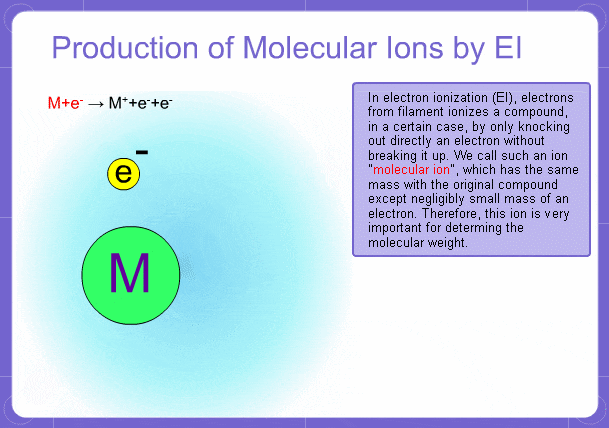
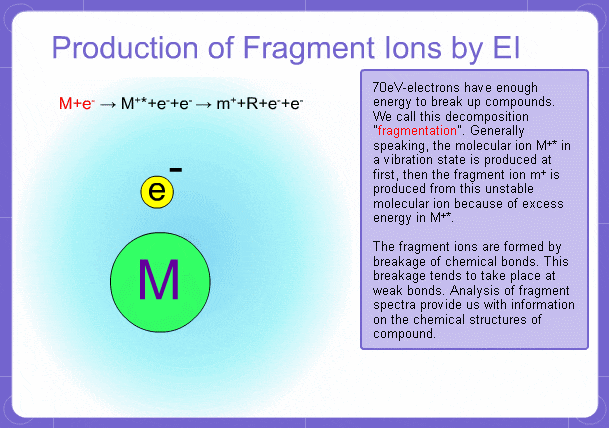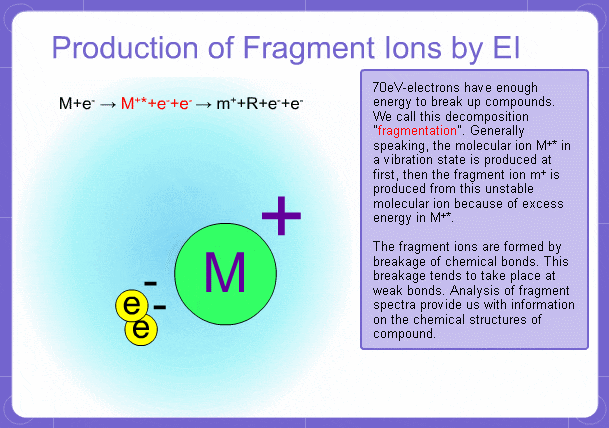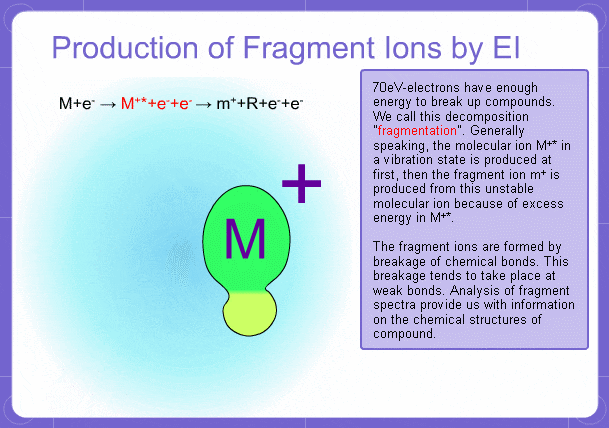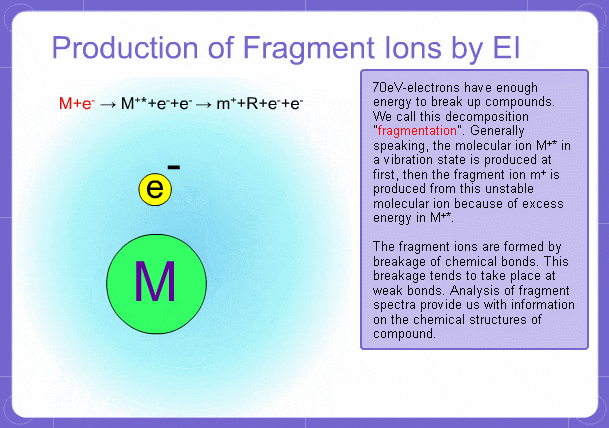
Sample components collide with fast electrons emitted from a filament and are thereby ionized. At the same time molecules are broken down. This breakage of molecules in mass spectrometry is called fragmentation. Therefore, with the EI method, molecular and fragment ions are created. Molecular weight is then determined from the molecular ions and structure is determined from the fragment ions. The original component in the sample can then be identified by combining the mass and structure information.
However, analysis of mass spectra is not easy and identification is generally done using data searches. In some cases, depending on the components, molecular ion peaks might not appear. In these cases, the CI method is used.
Molecular Ions
Fragment Ions
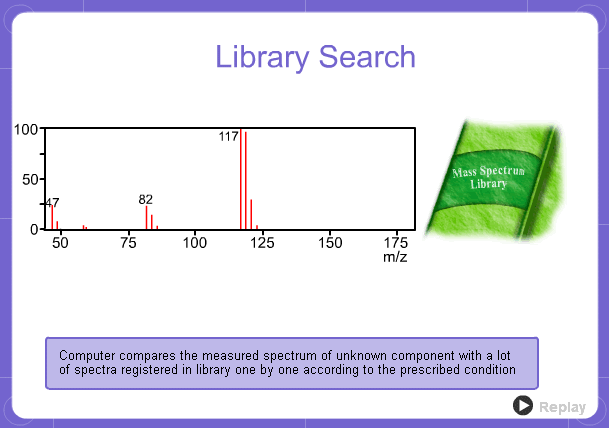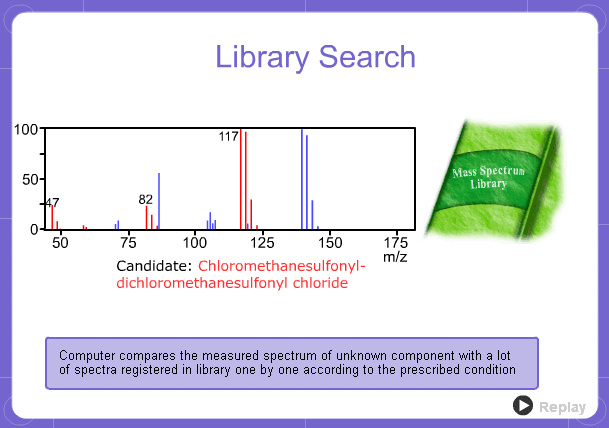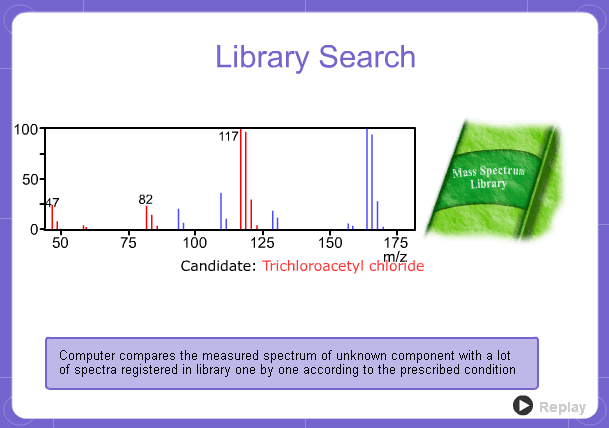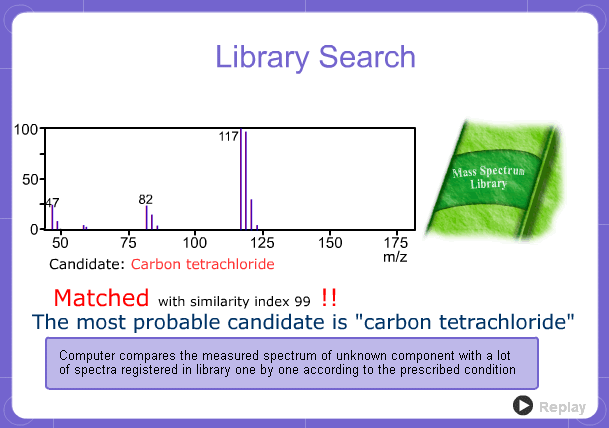
Library Search