Other Factors Affecting Separation
For HPLC, the column temperature affects separation only slightly, but for SFC, the temperature and pressure can affect the change in CO2 state, which changes the density, diffusion coefficient, and other properties of the CO2. Therefore, the temperature and pressure setting values are factors that can potentially affect separation (Fig. 1).
As explained in Analytical Advantages of SFC regarding the van Deemter equation, the large diffusion coefficient makes it easier for supercritical carbon dioxide to penetrate other substances, which results in a lower C value that is related to the mass transfer diffusion and enables higher separation efficiency. Increasing the temperature increases the diffusion coefficient and decreases the viscosity, which enables using a longer column and measuring samples at higher flow rates. Temperature and pressure mainly contribute to the number of theoretical plates, but the number of theoretical plates contributes to resolution by a factor equivalent to its square root. That means it does not have a large effect compared to modifiers, stationary phases, or other parameters that contribute to the selectivity of separation.
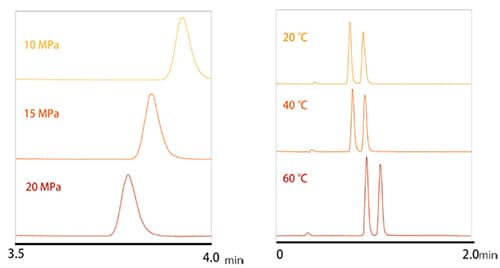
Fig. 1 Effects of Pressure and Temperature on SFC Analysis






