SFC Mobile Phases
When determining the mobile phase for HPLC, not only the solvent but also the acid/base level, the addition of ion-pair reagents or other modifiers, and other factors that help separation must be considered. For SFC, separation is affected by the quantity and type of modifier added, the acid or other additives added to the modifier, and the pressure and temperature applied to maintain the CO2 in a fluid state. CO2 is typically used as the mobile phase for SFC, but there are also cases of supercritical fluoroform being used.*1)
Modifiers
Due to the nonpolarity of supercritical carbon dioxide, a modifier such as methanol, ethanol, or acetonitrile can be added to provide polarity to the mobile phase. Increasing the modifier quantity could break down the supercritical fluid state of the CO2 and potentially change it to a subcritical or liquid state. Nevertheless, it will continue to function as a mobile phase regardless of the state and is not a problem for chromatography. Adding a modifier increases the critical temperature and critical pressure necessary for maintaining supercritical carbon dioxide hence adding high percentages of a modifier is not desirable. For HPLC, reversed-phase chromatography and normal-phase chromatography have symmetric properties that can counteract the ability of the mobile phase solvent to elute
components. For SFC, the supercritical carbon dioxide always has a low elution capability and the modifier has a high elution capability for both reserved-phase and normal-phase chromatography. Table 1 shows examples of solvents used as modifiers. Fig. 1 shows a comparison of chromatograms separated using different types of modifiers.
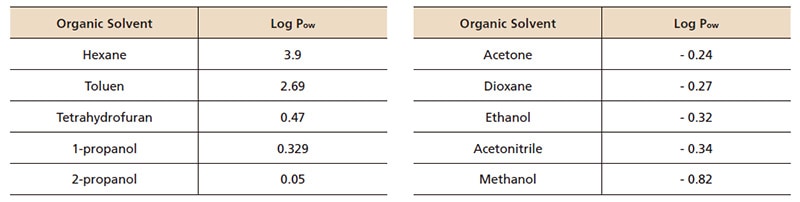
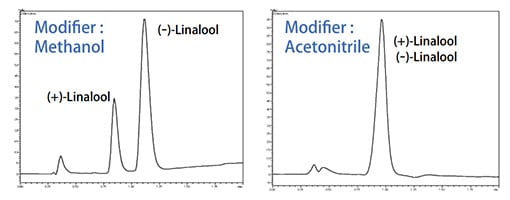
Fig.1 Comparison of Modifiers
Using Additives to Adjust Separation and/or Improve Peak Shape
For HPLC analysis, buffer solutions, ion-pair reagents, or other additives are used to change separation selectivity or improve peak shape. Similarly, additives are used for the same reasons in SFC as well.
Additives used for SFC are added to the modifier rather than the supercritical carbon dioxide. The main additives used are listed in Table 2. Adding an acid such as acetic acid or a base such as an amine can improve peak shape by inhibiting ionization of target components or by masking secondary functional groups in the stationary phase (Fig.2). For LC-MS detection, a volatile salt such as ammonium formate is often used because ion suppression can occur. It has been reported that adding a small amount of water ionizes part of the supercritical carbon dioxide so that it behaves like an acidic additive.*2)
Table2 Examples of Additives and Applicable Compounds
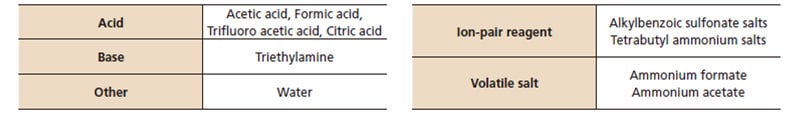
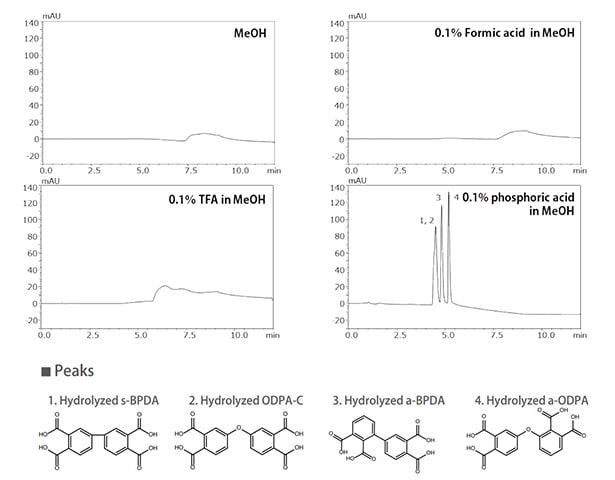
Fig. 2 Effect on Peak Shape of Salt or Acid Type Added to Modifier
Reference.
*1) Comparison of Reversed-Phase HPLC Separation Using Carbon Dioxide and Fluoroform for Enhanced-Fluidity Liquid Mobile Phases
Anal.Chem.1998,70,1595-1603
*2) Packed column supercritical fluid chromatography of hydrophilic analytes via water-rich modifiers
Journal of Chromatography A, Volume 1250, 10 Aug 2012, P196-204






