Example: For a 30 mg daily...
Co-Sense for Impurities
Liquid Chromatograph with online Sample Preparation

The FDA draft guidance, "Genotoxic and Carcinogenic Impurities in Drug Substances and Products: Recommended Approaches" prescribes permitted limits for genotoxic impurities in drug substances. These impurities demand more sensitive trace-level analysis than normal impurities. Generally, mass spectrometry methods, such as GC/MS or LC/MS, are used for the high-sensitivity analysis of impurities. However, there is increasing demand for the establishment of a high-sensitivity quantitation method using an absorbance or other conventional detector that is easy to operate and can easily apply existing LC analysis conditions.
Features
-
Impurity intake restricted to 1.5 µg/day max. when taking a drug over a long period (12 months or more)
Example: For a 30 mg daily... -
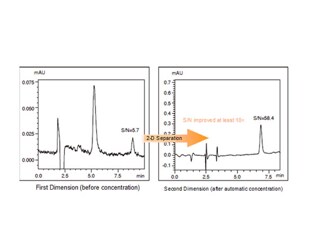
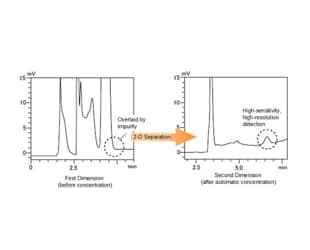
The Co-Sense for Impurities System achieves approximately 10 to 20 times higher sensitivity than 1-D separation only by...
-
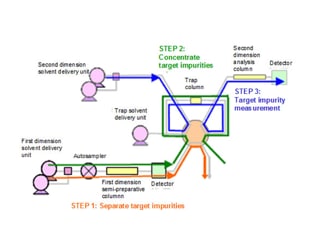
Using a 1-D and 2-D column with different retention characteristics, or using different mobile phase compositions, permits reliable separation and...
-
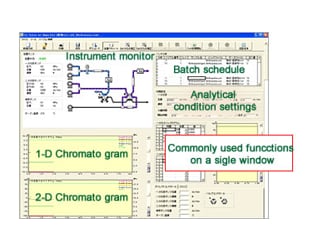
Significant knowledge and experience are generally required to set the analytical conditions and clean the flow lines of a 2-D separation system. However...
News / Events
-
Shimadzu has released the Shim-pack Mix-HILIC, Chromatography Column for Hydrophilic Interactions
Comprehensive Analysis of Hydrophilic Metabolites in Metabolomics
-
A new video has been released -Finding Out What Makes Things Sour-
Ever wondered what makes some foods sour? In this video we explore that question while also introducing one of Japan's traditional sour foods!
-
New Nexera FV
Nexera FV and LabSolutions™ FV together form a new UHPLC system capable of monitoring flow synthesis and batch synthesis reactions, and automating formulation dissolution testing.
-
Pharmaceutical and Biopharmaceutical has been updated.
Shimadzu Corporation has always been a reliable partner for the pharmaceutical industry. We provide comprehensive analytical solutions for all drug research analysis and productions and help companies to increase efficiency, reduce cost, and ensure drug safety and quality.
-
On-line extraction and determination of targeted carotenoids from habanero red (Capsicum Chinese)
The on-line coupling between supercritical fluid extraction (SFE) and supercritical fluid chromatography (SFC), generate a powerful tool for the automatic extraction and detection of target compounds from food matrices. The on-line nature of the system, compared to off-line
approaches, improves run-to-run precision, enables the setting of batch-type applications, and reduces the risks of sample contamination. Supercritical carbon dioxide (CO2) presents unique characteristics, which make it an excellent solvent, it shows a relatively high density and
consequently, a high solvation power. -
New Nexera UC Prep
The Nexera™ UC Prep is a new preparative supercritical fluid chromatography (SFC) system that offers both the high basic performance developed for the previous Nexera UC model and original state-of-the-art preparative SFC technologies. It resolves a number of issues in preparative tasks, reducing labor and improving efficiency while fitting into pre-existing workflows.