Nexera FV
Ultra High Performance Liquid Chromatograph for Online Analysis
Automates Online LC Analysis, from Sample Collection to Data Collection
Nexera™ FV

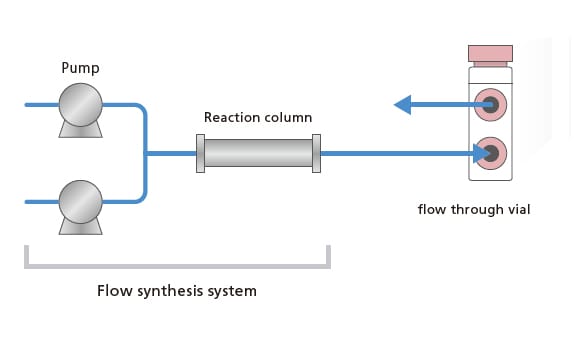
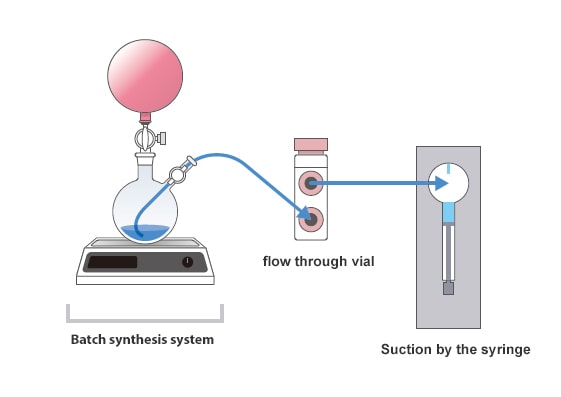
Nexera FV and LabSolutions™ FV together form a new UHPLC system capable of monitoring flow synthesis and batch synthesis reactions, and automating formulation dissolution testing.
From bulk drug to final product manufacturing, the Food and Drug Administration (FDA) and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) look for consistency in the manufacturing of pharmaceuticals. There is an increasing need for a Quality by Design (QbD) approach using process analytical technology (PAT), and process monitoring, in which the quality of each product is controlled during each unit operation. In measuring the critical quality attributes (CQA), Nexera FV and LabSolutions FV automate all the steps for PAT, including collection and dispensing of the reaction solution, HPLC analysis, data analysis, and the creation of reports. This achieves substantial labor savings and provides highly reliable data by eliminating human error.
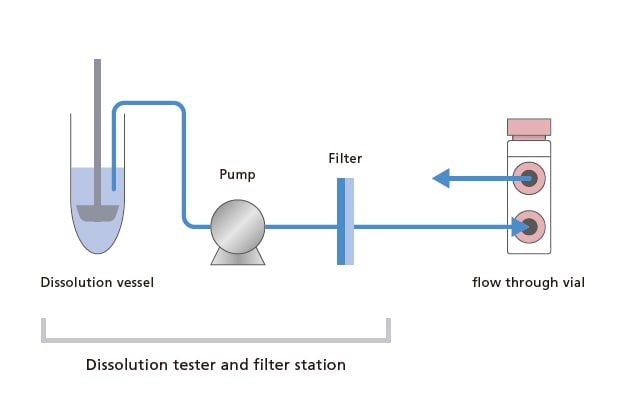
In dissolution testing as well, everything from periodic sample collection from the dissolution vessels to LC analysis is performed automatically, substantially reducing the burden on the analyst.
With its proprietary design, the Nexera FV flow through vial autosampler periodically collects samples that are sent into the flow through vial from the reaction tank or dissolution vessel. Two analytical modes can be selected depending on the sampling time and LC analysis time, to provide optimally timed analytical data collection. Further, the Nexera FV can also be used as a general-purpose UHPLC system, which contributes to increasing the asset's operating rate. Additionally, with just a few simple operations, LabSolutions FV implements settings for online analysis and data analysis for a wide range of applications, including process monitoring and dissolution testing.
Nexera FV and LabSolutions FV expand the possibilities for online LC, providing new solutions for pharmaceutical quality control.
Nexera and LabSolutions are trademarks of Shimadzu Corporation or its affiliated companies in Japan and/or other countries.
Features
News / Events
-
New Technical Report is available, Optimization of Supercritical Fluid Extraction Parameters for Vitamins D2, D3, and K1 from Pharmaceutical Preparations
New Technical Report is available, Optimization of Supercritical Fluid Extraction Parameters for Vitamins D2, D3, and K1 from Pharmaceutical Preparations
-
New Tips & Tricks is available, Automated Dilution and Preparation of Standard and Sample Solutions for Analysis
-
Shimadzu Corporation has released LabSolutions Detect, a software with AI Functionality to support Anomaly Detection for Liquid Chromatographs (LC).
LabSolutions Detect transforms your LC data review process by visualizing differences between accumulated reference data and daily sample data.
-
New Technical Report is available, A sustainable analytical approach for detecting extra virgin olive oil adulteration using Nexera™ UC
In this study, a fast, simple and green methodology was optimized to detect intentionally adulterated extra virgin olive oil EVOO with cheaper seed oils at different levels by means of subcritical fluid chromatography (subFC) with UV detection, followed by statistical analysis.
-
Shimadzu has released the new integrated LC system, i-Series.
The new i-Series integrated LC: Sustainable design. Reliable results. Uncompromising performance.
-
Core-shell Column: Analysis Basics Now Available.
Discover easy-to-understand insights into the fundamentals of analysis.