What does 𝑚/𝑧 mean?
Basics of Mass Spectrometry
What is Mass Spectrometry?
Mass spectrometry (MS) is a technique used to identify and quantify molecules based on their mass-to-charge ratio (m/z). It helps determine the molecular weight and structure of compounds.
What does m/z mean?
M stands for mass and Z stands for charge number of ions. In mass analysis, an electron is taken from molecules to create single charged ions. If two electrons are removed, double charged ions are produced. The number of electrons removed is the charge number (for positive ions). m/z represents mass divided by charge number and the horizontal axis in a mass spectrum is expressed in units of m/z. Since z is almost always 1 with EI on GCMS, the m/z value is often considered to be the mass.
For example, if a molecule has a mass of 100 and a charge of +1, the m/z = 100. If it has a charge of +2, the m/z = 50.
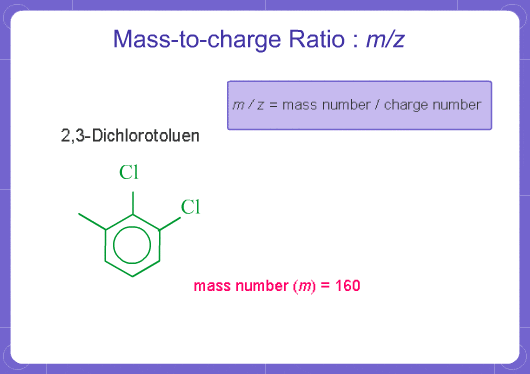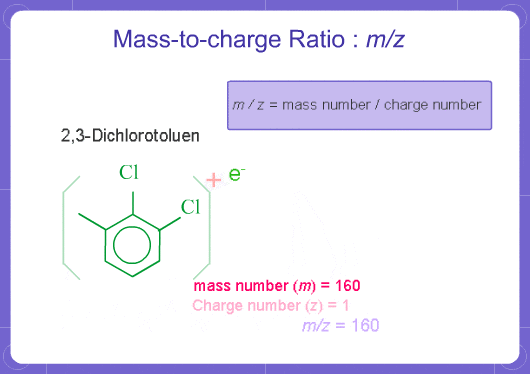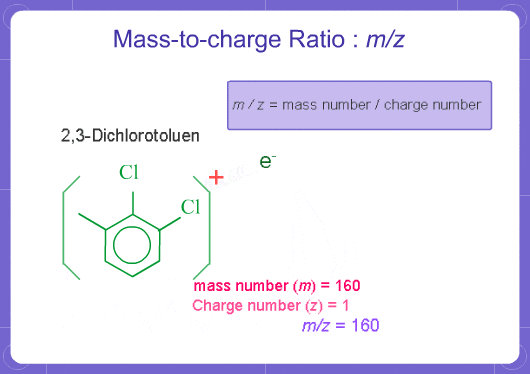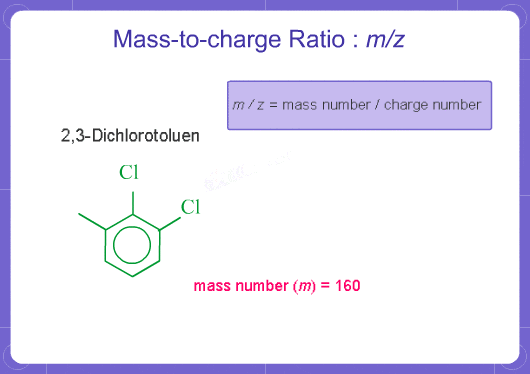
How Does Mass Spectrometry Work?
-
Ionization: Molecules are charged using methods like Electron Ionization (EI) or Electrospray Ionization (ESI).
-
Acceleration: Ions are accelerated in an electric field.
-
Separation: Ions are separated based on m/z in a mass analyzer (e.g., quadrupole, TOF).
-
Detection: A detector records the ions, generating a mass spectrum.
-
Analysis: The resulting spectrum is interpreted to determine molecular structure and quantity.
Components of a Mass Spectrometer
-
Ion Source: Where molecules are charged (e.g., by losing an electron).
-
Mass Analyzer: Sorts ions based on m/z.
-
Detector: Measures ion abundance and outputs the data.
FAQ
What is mass spectrometry used for?
Mass spectrometry is used to identify and quantify chemical compounds by analyzing their mass-to-charge (m/z) ratio. It’s widely applied in pharmaceuticals, environmental testing, forensics, and proteomics.
What does m/z mean in mass spectrometry?
m/z stands for mass-to-charge ratio. It represents a particle’s mass divided by its charge number and is the primary value plotted in a mass spectrum.
What are the main components of a mass spectrometer?
A mass spectrometer typically includes:
-
Ion source (to charge molecules)
-
Mass analyzer (to separate ions by m/z)
-
Detector (to record ion intensity and abundance)
How does a mass spectrometer work?
Mass spectrometry involves ionizing molecules, accelerating them, separating them by m/z, detecting them, and analyzing the resulting spectrum to identify or quantify substances.






