MultiNA II MCE-301
Microchip Electrophoresis System
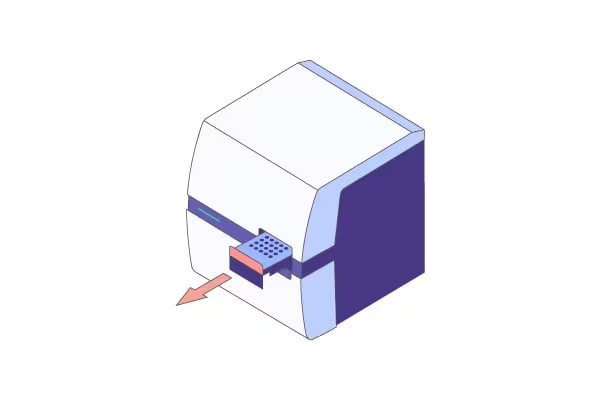
The MultiNA II MCE-301 streamlines genetic analysis workflows by fully automating nucleic acid electrophoresis—a process that typically requires over two hours of manual work.
Simply register your samples, load the dedicated reagents and samples into the instrument, and start the analysis. The system also supports adding samples during ongoing runs, offering significantly improved operability compared to the previous model (MCE-202 MultiNA).
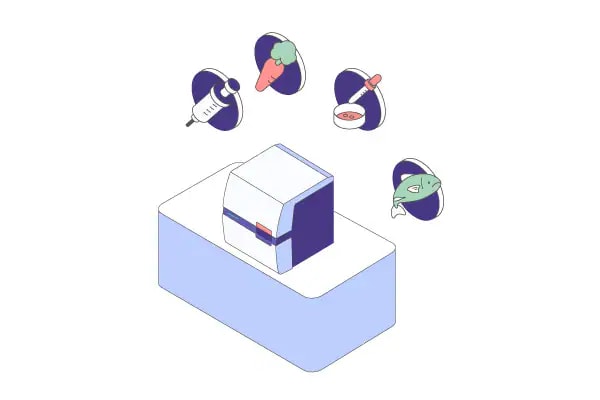
In addition, new analysis functions enable a wide range of applications, including quality checks for next-generation sequencing libraries and total RNA, verification of mRNA purity, and mutation detection for genome editing, as well as various genotyping tasks.
MultiNA is a trademark of Shimadzu Corporation or its affiliated companies in Japan and/or other countries.
Features
Videos
-
MultiNA II MCE-301, Microchip Electrophoresis System
News / Events
-
The MultiNA II MCE-301, Microchip Electrophoresis System has been released
The MultiNA II MCE-301 streamlines genetic analysis workflows by fully automating nucleic acid electrophoresis—a process that typically requires over two hours of manual work.
-
CASE STUDIES: "Lipid research at UT Southwestern could revolutionize diabetes treatment" is now available.
Can the science of fats unlock a new avenue in diabetes care? UT Southwestern researchers are finding out.
-
New IMAGEREVEAL MS, Mass Spectrometry Imaging Data Analysis Software
Automatically uncover important information from large amounts of data with IMAGEREVEAL MS.
-
New iMScope QT
Inheriting the concept of a mass spectrometer equipped with an optical microscope from the iMScope series, the iMScope QT is also Shimadzu's flagship model for MS imaging with a LCMS-Q-TOF.
-
Shimadzu has released the Cell Pocket Ver.2.10 Web Application Supporting Cellular Observations
Cell Pocket is an integrated system for both the analysis and management of cell images.
-
Shimadzu has released the Tm Analysis System
Tm analysis systems can accelerate the development process and improve the quality of oligonucleotide therapeutics. Control by LabSolutionsTM software enables compliance with ER/ES-related regulatory requirements and improves the efficiency of analyzing the thermal stability (Tm analysis) of nucleic acids.