SFC Analytical Condition Parameters
The approach for the SFC analytical condition development process is basically the same as for determining analytical conditions for HPLC. HPLC can generally target any component in compounds that can be dissolved in the mobile phase. In contrast, SFC can be used to analyze any compound that is compatible with supercritical carbon dioxide and can be dissolved in an organic solvent.
If chromatography is used for quantitative analysis, then optimal analytical conditions should achieve resolution of 1.5 or more. The basic equation for calculating resolution Rs is indicated below.
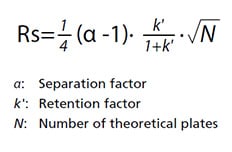
The α, k, and N values that affect Rs are all independent factors that vary depending on the column and mobile phase used, where N represents separation efficiency and α selectivity. In particular, separation can be improved by increasing N and α values.
Because the supercritical carbon dioxide used for SFC offers different properties than mobile phase solvents used for HPLC, when a column used for HPLC is used for SFC, it can sometimes result in different elution behavior (Fig. 1). The following chapter describes the characteristics of columns and mobile phases involved in determining analytical conditions for SFC.
Learn More
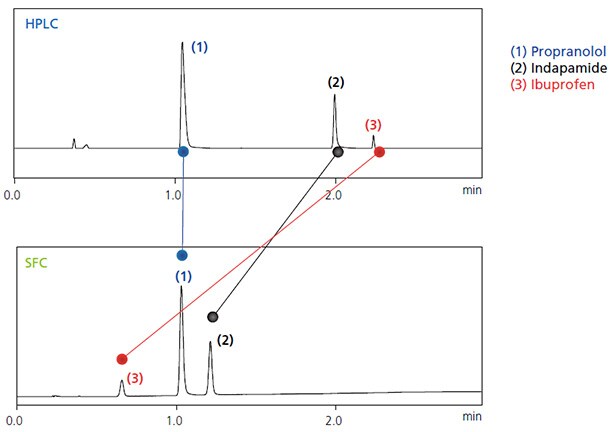
Fig. 1 Separation of Three Types of Pharmaceutical Ingredients (Upper: HPLC; Lower: SFC)






